A prospective Phase 2 clinical trial, called NOVEMBER (NCT3345420), has provided compelling evidence for a novel, ultra-short, 9-day course of whole breast radiation therapy. This regimen, which includes a simultaneous boost to the lumpectomy cavity, demonstrated excellent local cancer control and high patient satisfaction while significantly reducing the time patients spend in treatment.
For many women, particularly those living in rural areas with long travel times to a cancer center, convenience is a major factor in treatment adherence. Standard radiation schedules have historically stretched over 6 to 8 weeks. While modern accelerated hypofractionation has shortened this, the NOVEMBER trial sought to create a comprehensive, two-week schedule that could make treatment more accessible and less burdensome.
Trial Design and Methodology of NOVEMBER Trial
The trial was a single-arm, Phase 2 study that enrolled 103 women over the age of 18 with anatomically staged Tis and T1-3N0 breast cancer. Patients were required to have negative surgical margins and were recommended for a lumpectomy cavity boost by their radiation oncologist. Exclusion criteria included a history of prior chest radiation, active collagen vascular disease, or significant post-lumpectomy complications.
The treatment plan involved delivering 34.2 Gy to the entire breast and 39.6 Gy to the lumpectomy cavity, all delivered in just 9 consecutive daily treatments, excluding weekends. The trial’s primary endpoint was the photographic cosmetic score at 24 months post-radiation, with a goal of proving non-inferiority by achieving a good-to-excellent score in over 70% of patients.
Secondary endpoints included monitoring for acute and late-onset radiation toxicities based on the NCI’s CTCAE criteria and assessing patient-reported outcomes (PROs) using the validated Breast-Q survey to measure breast satisfaction and well-being.
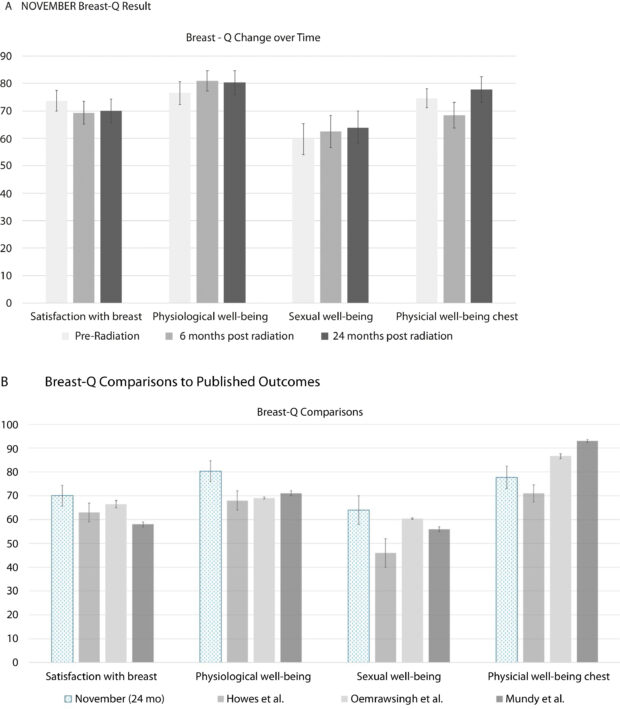
(a) NOVEMBER Trial Breast-Q results. (b) Breast-Q comparisons to published outcomes. Figures have been shown with 95% confidence intervals. Howes et al43 lumpectomy patients, Oemrawsingh et al42 breast surgery only patients, Mundy et al44 nonbreast cancer “normal,” population.
Read About Novel Long Non-Coding RNA Boosts Radiotherapy Efficacy in Lung Cancer
Results and Clinical Findings
With a mean follow-up of 51 months, the trial’s most significant clinical finding was its excellent local cancer control, with zero local breast cancer recurrences reported. This compares favorably with the recurrence rates seen in longer, established trials.
- Cosmetic Outcomes: The trial’s primary cosmetic endpoint was not met, as 68% of the 87 evaluable patients were found to have an excellent/good result, falling just shy of the 70% goal. However, the researchers noted that their hypothesis was based on a prior trial that did not include a boost, which is known to worsen cosmetic outcomes. A more positive finding was that 80% of all patients experienced either an improvement or no noticeable cosmetic change from their baseline photos.
- Patient Satisfaction: Patient-reported outcomes were highly positive. On the Breast-Q survey, 85% of women reported being satisfied with their breasts 24 months after treatment.
- Toxicities: The regimen was very well-tolerated. There were no late toxicities of Grade 3 or higher. Only four patients experienced a late Grade 2 toxicity, such as chest wall pain or skin tightness, while acute Grade 2 or 3 events (mainly dermatitis and breast pain) were more common but resolved after treatment.
Discussion and Future Directions
The NOVEMBER trial successfully proves that its 9-day radiation schedule with a simultaneous boost is a safe and highly effective approach. The researchers acknowledge that their initial cosmetic goal was perhaps too ambitious, noting that a more realistic comparison would have been to the EORTC boost trial, which reported a 71% good/excellent outcome.
The trial’s success in achieving excellent local control and high patient satisfaction has set the stage for a crucial next step. The researchers plan to launch a Phase 2 randomized trial that will directly compare the 9-day NOVEMBER schedule against a 5-day “Fast Forward” schedule, with both regimens including a simultaneous boost. This will be the first randomized trial to compare two different accelerated SIB schedules. The researchers hypothesize that the 9-day course may offer a superior patient experience and cosmetic results, as prior data suggests an overly compressed 5-day schedule may lead to worse outcomes for some patients.
Read Full Artilce On: International Journal of Radiation Oncology, Biology, Physics
Written By Aren Karapetyan, MD


