Original Study Title: Long Non-Coding RNA LOC401312 Induces Radiosensitivity Through Upregulation of CPS1 in Non-Small Cell Lung Cancer
Authors: Zhengyue Cao, Tiantian Wang, Fumin Tai, Rui Zhai ,Hujie Li, Jingjing Li, Shensi Xiang, Huiying Gao, Xiaofei Zheng, Changyan Li
Long non-coding RNAs are non-protein-coding genetic transcripts that play crucial roles in regulating gene expression. While radiation therapy works by damaging cancer cell DNA, the exact involvement of lncRNAs in this process has been poorly understood. This study aimed to systematically identify lncRNAs that influence radiation response in NSCLC.
For years, much of our genome was considered “junk DNA” because it didn’t code for proteins. However, modern science has revealed that non-coding RNAs, especially lncRNAs, are crucial regulators of gene expression, influencing everything from cell development to disease responses. While radiation therapy is a cornerstone of cancer treatment, inducing cellular damage by breaking DNA, the specific ways lncRNAs influence a cell’s response to radiation have remained largely uncharacterized. This study aimed to systematically pinpoint lncRNAs involved in NSCLC’s response to radiation.
Discovery of a Novel Radiosensitizing Pathway
Using genome-wide CRISPR activation (CRISPRa) screening in NSCLC cells (specifically A549 and H460 lines), researchers meticulously searched for lncRNAs that could alter how these cancer cells react to ionizing radiation. Among thousands, LOC401312 emerged as a critical player. Its stable overexpression significantly enhanced the cells’ susceptibility to radiation.
Transcriptomic profiling revealed the core mechanism: LOC401312 exerts its effect by transcriptionally upregulating carbamoyl-phosphate synthase 1 (CPS1). CPS1 is a mitochondrial enzyme known for its role in pyrimidine biosynthesis and the detoxification of ammonia in the urea cycle. This study found that LOC401312 increased CPS1 mRNA levels by an impressive 37.25-fold in A549 cells and 27.88-fold in H460 cells, with a corresponding increase in CPS1 protein.
Crucially, the study directly validated CPS1’s independent role in radiosensitization. Overexpressing CPS1 alone in lung cancer cells significantly impaired their proliferation after radiation and strikingly reduced their ability to form colonies—by 72.2% in A549 cells and 52.7% in H460 cells. This confirmed that the radiation-sensitizing effects of LOC401312 are functionally dependent on CPS1. Attempts to completely remove CPS1 from cells were largely infeasible, as its depletion profoundly inhibited basic cell growth, underscoring its vital cellular role.
Further mechanistic investigations pinpointed how this axis works: CPS1 was found to suppress the phosphorylation of ATM kinase (Ser1981). ATM is a master regulator of the DNA damage response (DDR) pathway, orchestrating cell cycle checkpoints and DNA repair. By suppressing ATM activation, the LOC401312–CPS1–ATM axis impairs the cell’s ability to effectively repair radiation-induced DNA damage, leaving the cancer cells vulnerable.
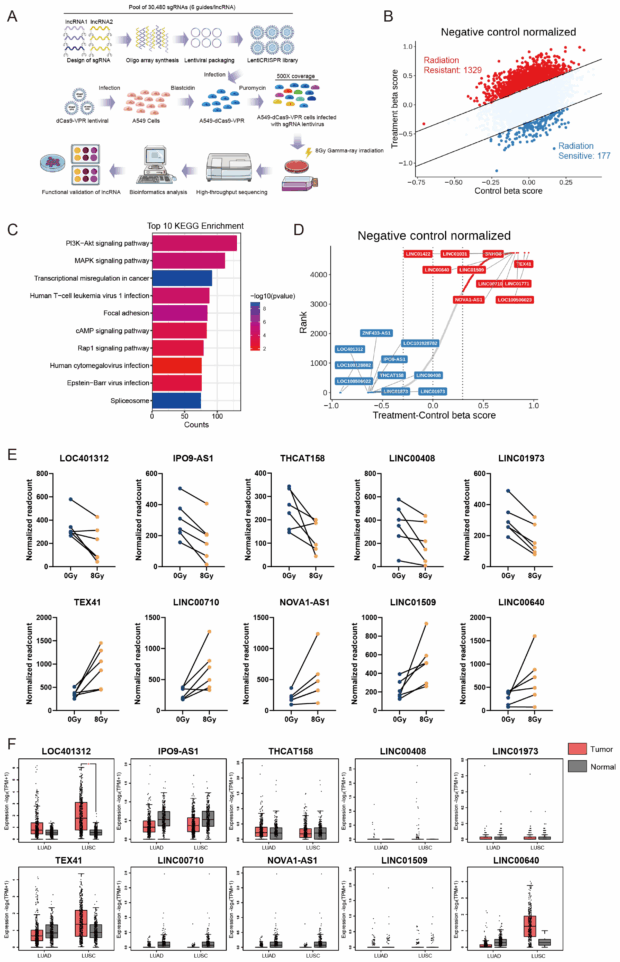
Systematic Identification of Radiation-Responsive lncRNAs via CRISPRa Screening in NSCLC. (A) Schematic of the CRISPRa high-throughput library screening strategy for identifying radiation-responsive lncRNAs in A549 cells, comprising 30,480 sgRNAs targeting 4766 lncRNAs (6 sgRNAs per gene) with >500× coverage per sgRNA. (B) Scatter plot of Delta-beta scores for screened lncRNAs. (C) KEGG pathway analysis and protein interaction network of candidate lncRNAs predicted using the LncSEA database (http://bio.liclab.net/LncSEA, accessed on 1 November 2021).
(D) Delta-beta score ranking and differential gene visualization of identified lncRNAs. (E) Enrichment analysis of sgRNAs targeting lncRNAs, showing significant depletion of all six sgRNAs (normalized read counts shown). (F) Differential lncRNAs expression in normal tissues versus lung adenocarcinoma (LUAD) and squamous cell carcinoma (LUSC) clinical samples using the GEPIA2 database (http://gepia2.cancer-pku.cn, accessed on 10 June 2025). * p < 0.05 by unpaired two-tailed Student’s t-test.
Read About Proton Therapy of IDH-Mutant Gliomas
Clinical Implications and Future Outlook of Long non-coding RNAs
This study definitively establishes the LOC401312–CPS1–ATM axis as a previously unrecognized regulatory network governing radiation sensitivity in NSCLC. This discovery not only expands our fundamental understanding of how lncRNAs influence cellular responses to DNA damage but also provides a strong therapeutic rationale for clinical intervention.
Targeting the LOC401312–CPS1 pathway represents a promising new strategy to improve the efficacy of radiotherapy for non-small cell lung cancer. CPS1 emerges as a dual-functional candidate: it could serve as a potential diagnostic and prognostic biomarker for predicting radiation response and also as a direct therapeutic target to make tumors more susceptible to existing radiation treatments. While these findings provide robust mechanistic insights from in vitro models, the next critical step involves in vivo validation through preclinical models and patient-derived xenograft systems. This will help delineate the precise molecular mechanisms further and explore the dynamics of urea cycle metabolites in a clinically relevant context, ultimately bringing this research closer to benefiting NSCLC patients.
You Can Read Full Article on: International Journal Of Molecular Sciences
Written By Aren Karapetyan, MD