Original Study Title: Proton therapy for adult type IDH-mutated glioma: Proglio-1, a multicenter retrospective study
Authors: Nicolas Goliot, Emmanuel Jouglar, Julian Jacob, François Christy, Eva Seutin, Renaud Schiappa, Sarah Dumont, Selim Mohssine, Dinu Stefan, Arthur Leclerc, Evelyne Emery, Cyril Moignier, Jeanne Riverain, Fernand Missohou, Maxime Fontanilles, Samuel Valable, Jacques Balosso, Jérôme Doyen & Paul Lesueur
IDH-mutant gliomas in adults are showing encouraging mid-term survival and a favorable tolerance profile with proton therapy, a precision radiation technique that spares healthy tissue, according to a multicenter French study. This offers a vital treatment option for younger adults battling these brain tumors.
Gliomas with IDH mutations often affect younger individuals who have a long life expectancy. While traditional photon radiotherapy is effective, concerns have grown regarding its potential detrimental effects on long-term cognition and quality of life due to its broader dose distribution to healthy brain tissue. Proton therapy, with its precise “Bragg peak” energy delivery, aims to minimize this collateral damage. Although no prospective clinical trial has yet directly compared front-line photon versus proton therapy for survival and cognitive impact, ongoing studies are anticipated to yield results in the coming years.
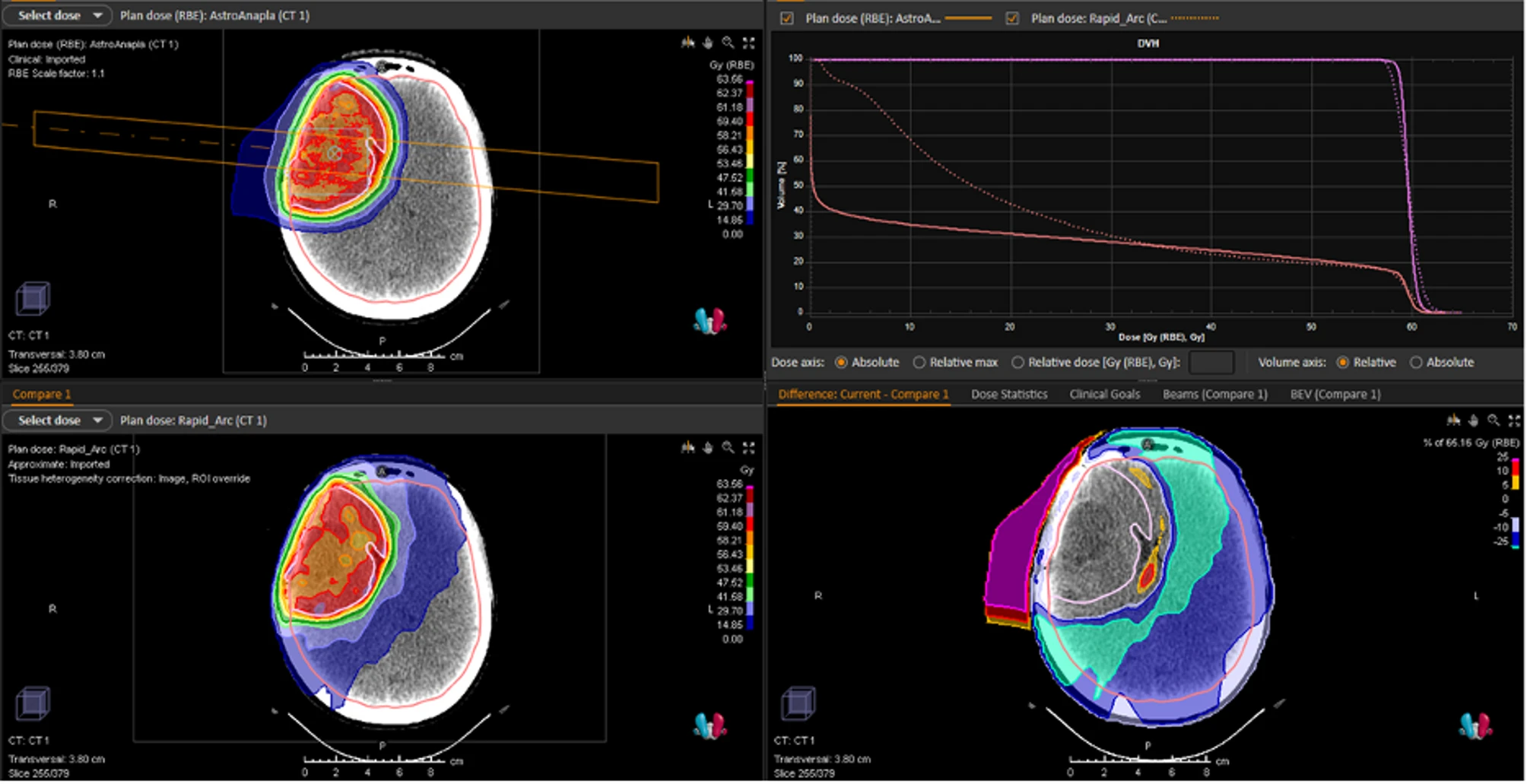
Dosimetric comparison of proton therapy (SFUD) versus VMAT for IDH1-mutated astrocytoma. Legend: Dosimetric comparison of Single-Field Uniform Dose (SFUD) proton therapy irradiation (upper left) with a photonic VMAT plan (lower left) for the treatment of IDH1-mutated anaplastic astrocytoma. Illustration of the benefit of proton therapy on the Dose Volume Histogram (upper right); difference in dose distribution (lower right)
Comprehensive French Multicenter Study on IDH-Mutant Gliomas
This retrospective, multicenter study was conducted across the three French proton therapy centers (Caen, Nice, and Orsay). The research was approved by the Ethics Committee of the François Baclesse Comprehensive Cancer Center and adheres to the Declaration of Helsinki. It specifically focused on adult patients (over 18 years old) with a histologically proven diagnosis of oligodendroglioma or IDH-mutated astrocytoma, irrespective of grade. Patients were treated with proton therapy between 2016 and 2024.
The gross tumor volume (GTV) was meticulously defined using T1-weighted MRI with contrast enhancement or T2 FLAIR sequences. The clinical target volume (CTV) was generated by expanding the GTV or the postoperative residual cavity by 10–15 mm, adjusted to anatomical boundaries, with a 95% prescription isodose line for robust coverage. Patients were regularly followed with clinical examinations and MRI.
Patient and Treatment Characteristics
The study cohort comprised 90 patients, with a median age at initial diagnosis of 36 years (range: 21–65 years). The majority (94%) had IDH1-mutated gliomas. Sixty-seven percent (60 patients) were diagnosed with IDH-mutant astrocytoma (28% Grade 2, 26% Grade 3, 3% Grade 4), while 33% (30 patients) had oligodendroglioma (14% Grade 2, 10% Grade 3). At the time of proton therapy, WHO grades 2, 3, and 4 were observed in 42%, 54%, and 3% of patients, respectively.
Regarding treatment, 46% of patients (41 individuals) received proton therapy as an upfront intervention, with the remaining 54% (49 patients) receiving it following recurrence, either exclusively or after additional surgery. Most Grade 2 glioma patients (87%) received a total dose of 50.4–54 Gy (RBE), while 83% of Grade 3–4 glioma patients received 59.4–60 Gy (RBE). Pencil Beam Scanning (PBS) was the predominant delivery method (78% of patients). Many patients had received prior chemotherapy, particularly those treated for recurrence.
Key Findings: Favorable Outcomes and High Tolerance
After a median follow-up of 27.3 months (range: 1.54–97.1 months) post-proton therapy, the study yielded positive mid-term results:
- Overall Survival (OS): The median OS for the entire cohort was not reached, indicating excellent survival rates thus far. OS rates were 98% at one year, 92% at two years, and 88% at three years post-PT. While no significant difference was seen based on histology type, a trend favored oligodendroglioma (2-year OS rates: 88% for astrocytoma vs. 100% for oligodendroglioma). Similarly, 2-year OS rates were 92% for both WHO grade 2 and grades 3–4. Patients who received proton therapy as upfront treatment showed significantly better OS than those treated at recurrence (p = 0.017), with the upfront group maintaining 100% OS at two and three years.
- Progression-Free Survival (PFS): The median PFS for the whole cohort was 42.5 months. PFS rates were 90% at one year, 75% at two years, and 62% at three years post-PT. PFS was significantly shorter for WHO grades 3–4 gliomas (37.3 months) compared to WHO grade 2 (median PFS not reached; p = 0.044).
- Prognostic Factors: Univariate analysis identified prior chemotherapy lines as the only factor significantly associated with worse PFS. The timing of PT (upfront vs. recurrence) was significantly associated with OS, favoring upfront treatment.
- Recurrence Patterns: A total of 28 patients (31%) experienced progression. The vast majority (79%) relapsed in-field (within the 95% isodose area), suggesting effective tumor coverage by proton therapy. Out-of-field relapses were rare (7%), and mixed patterns were observed in 11%. The predominant in-field recurrence pattern highlights proton therapy’s effectiveness in tumor coverage and may facilitate re-irradiation options for out-of-field progression.
- Radiation-Induced Contrast Enhancement (RICE): RICE was observed in 26% of patients (30% pseudoprogression, 70% radionecrosis), with a median onset of 11 months post-PT. Reassuringly, 87% of these cases were asymptomatic, and only 26% required treatment, such as corticosteroids or bevacizumab. Distinguishing RICE from true tumor progression remains a challenge due to overlapping radiological features.
- Tolerance and Toxicity: Proton therapy demonstrated an excellent safety profile.
- Acute Toxicities (<6 months): Primarily mild (Grade 1: 66%, Grade 2: 28%). Common symptoms included alopecia (80%), radiation dermatitis (46%), asthenia (39%), nausea/vomiting (39%), and headache (33%). Only three Grade 3 toxicities (3%) were reported, all being nausea/vomiting.
- Late Toxicities (>6 months): Uncommon and mild (Grade 1–2 only). Most frequent were asthenia (24%) and alopecia (16%).
- Neurocognitive Side Effects: Reported in 7% of patients acutely (mostly Grade 1) and 16% long-term (mostly Grade 1), based on physician notes. However, most patients did not undergo comprehensive neuropsychological examinations, a recognized challenge in routine clinical practice.
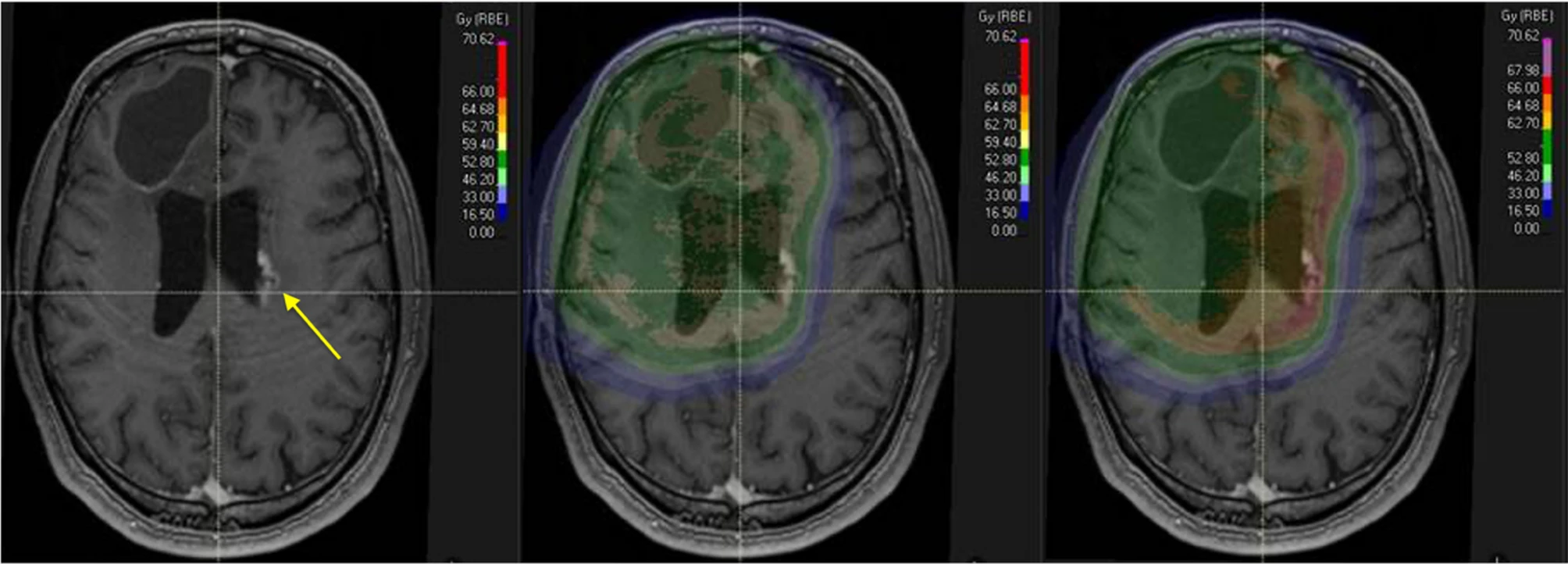
Imaging and dose distribution of radionecrosis under fixed and variable RBE models. Legend: Typical imaging characteristics of radionecrosis (yellow arrow on the left) with a periventricular distribution.
Implications: Preserving Quality of Life for Young Adults
The findings underscore the potential of proton therapy as a crucial component in the multimodal management of IDH-mutated gliomas. Given that these tumors primarily affect healthy young adults with long life expectancies, prioritizing the preservation of neurocognitive functions and overall quality of life is paramount.
While traditional photon radiotherapy techniques have advanced, they still result in a broad distribution of low and intermediate radiation doses throughout the brain, which can contribute to long-term side effects such as cognitive impairment, cerebrovascular events, and even secondary radiation-induced cancers. Proton therapy’s unique Bragg peak property allows for a more precise dose delivery, significantly minimizing radiation exposure to surrounding healthy brain tissue. This precision is why IDH-mutated gliomas are considered a potentially relevant indication for proton therapy in France.
Challenges and Future Directions
Direct comparison with conventional RT remains challenging due to the heterogeneity of patient populations and treatment protocols across studies. The survival rates observed in this cohort were lower than some historical photon RT trials, which the authors attribute in part to the inclusion of patients who received proton therapy after recurrence (54% of the cohort), which naturally skews survival data.
This study, as the first of its kind in France for adult IDH-mutated gliomas, provides valuable mid-term data. However, as a retrospective analysis, it has inherent limitations, including potential classification biases due to a lack of centralized pathology review and a follow-up period that may be insufficient to assess very long-term disease control and late toxicities.
Future large-scale randomized controlled trials, such as NCT05190172, APPROACH, and NRG-BN005, are currently underway to directly compare photon and proton therapy in terms of survival outcomes, quality of life, and neurocognitive toxicities for IDH-mutated WHO grade 2–3 gliomas. Until these long-awaited results become available, the findings of this study offer important insights. Furthermore, the excellent tolerance profile and predominantly in-field recurrence pattern observed suggest that future research into dose escalation strategies with proton therapy could be a relevant area for optimizing local control while continuing to preserve healthy tissues.
You Can Read Full Article on : BMC Radiation Oncology
Written By Aren Karapetyan, MD


