Brain cancer refers to the growth of abnormal cells within the brain, and it can arise from both primary and secondary sources. Primary brain tumors originate in the brain, while secondary brain tumors, also known as metastatic brain tumors, occur when cancer spreads from another part of the body to the brain.
Types of Brain Cancer
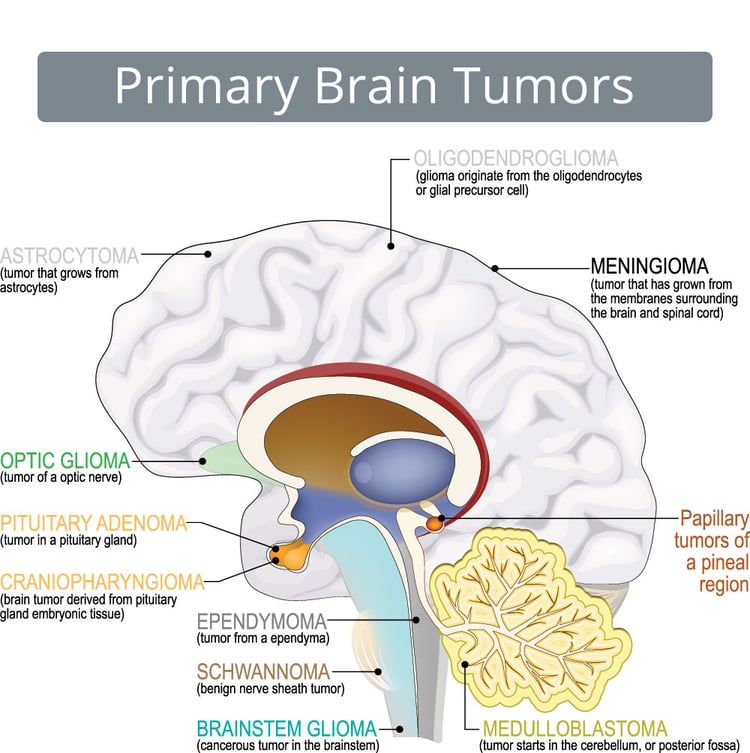
Brain cancers are broadly categorized into primary (originating in the brain) and secondary (metastatic, originating elsewhere and spreading to the brain). Primary brain tumors are further divided based on the cell of origin and biological behavior, either benign or malignant. Below is an expanded explanation of the most recognized malignant types of primary brain cancers.
Astrocytoma
Astrocytomas span a wide range of grades (I to IV).
- Pilocytic astrocytoma (Grade I) is usually benign, slow-growing, and often curable with surgery, commonly seen in children.
- Diffuse astrocytoma (Grade II) grows slowly but can progress to higher-grade tumors.
- Anaplastic astrocytoma (Grade III) is malignant and often progresses to glioblastoma.
The molecular profile, including IDH mutation status, is now integral in classification and prognosis (Louis et al., 2021).
Glioblastoma or Grade IV Astrocytoma
Glioblastoma is the most aggressive and common primary malignant brain tumor in adults. It arises from astrocytes, star-shaped glial cells that support neurons. These tumors are highly infiltrative, making surgical resection challenging, and they tend to recur despite aggressive treatment. Glioblastomas often present with rapid neurological decline, headaches, and seizures. The prognosis remains poor, with median survival around 12–15 months despite maximal therapy including surgery, radiotherapy, and temozolomide chemotherapy (Stupp et al., 2005; Ostrom et al., 2022).

Read More About Glioblastoma on Oncodaily
Oligodendroglioma
This tumor arises from oligodendrocytes and typically occurs in adults aged 30–50. Characteristically slower-growing than glioblastoma, oligodendrogliomas often present with seizures. The presence of 1p/19q codeletion and IDH mutation defines the molecular subtype and is associated with better prognosis and sensitivity to chemotherapy, particularly PCV (procarbazine, lomustine, vincristine) (van den Bent et al., 2013).
Ependymoma
These tumors arise from ependymal cells lining the ventricles and central canal of the spinal cord. They are more common in children and are usually located in the posterior fossa (brainstem and cerebellum). Ependymomas can be slow or fast-growing depending on grade, and their treatment includes surgical resection and radiotherapy. Molecular subtypes (such as RELA fusion-positive ependymomas) affect prognosis (Pajtler et al., 2015).
Medulloblastoma
A highly malignant embryonal tumor, medulloblastoma is most common in children and originates in the cerebellum. Despite its aggressiveness, it is highly responsive to treatment. Standard therapy includes surgery, craniospinal irradiation, and chemotherapy. Molecular classification (WNT, SHH, Group 3, and Group 4) now guides treatment and prognosis, with WNT subtype having the best outcome (Taylor et al., 2012).
Primary Central Nervous System Lymphoma
This rare type of brain cancer is usually a non-Hodgkin B-cell lymphoma that arises in immunocompromised patients or elderly individuals. PCNSL typically presents with focal neurological deficits, cognitive changes, or seizures. High-dose methotrexate is the mainstay of treatment, often combined with rituximab and/or radiotherapy. Prognosis depends on patient age, immune status, and response to therapy (Batchelor & Loeffler, 2006).
Brain Metastases
While not a primary tumor, brain metastases are the most common brain malignancies. They originate from cancers such as lung, breast, melanoma, and renal cell carcinoma. Metastases may present with headaches, seizures, or focal neurological symptoms. Treatment often involves a combination of surgery, stereotactic radiosurgery, and systemic therapies targeted to the primary cancer (Gavrilovic & Posner, 2005).
Staging of Brain Cancer
Unlike many other malignancies, brain tumors, including both primary and metastatic cancers, are not staged using the traditional TNM (Tumor, Node, Metastasis) system due to the unique anatomy of the central nervous system and the infrequent spread to lymph nodes. Instead, brain cancers are typically categorized based on their grade, location, size, extent of invasion, and presence of metastasis within the central nervous system.
World Health Organization Grading System
The WHO classifies brain tumors into four grades (I–IV) based on histological features:
- Grade I: Benign and slow-growing, often curable with surgery (e.g., pilocytic astrocytoma).
- Grade II: Low-grade but infiltrative tumors with potential for recurrence and progression (e.g., diffuse astrocytoma).
- Grade III: Malignant, more aggressive, and likely to recur (e.g., anaplastic astrocytoma).
- Grade IV: Highly malignant with rapid progression and poor prognosis (e.g., glioblastoma).
Extent of Disease
Physicians also describe brain cancer according to its spread within the brain and spinal cord:
The progression of brain cancer is often categorized by how widely the tumor has spread within the central nervous system. Localized brain tumors remain confined to a single, defined area of the brain without infiltrating neighboring tissues. Diffuse or infiltrative tumors extend into surrounding brain structures, making complete surgical removal challenging. Multifocal tumors present as multiple, separate areas of disease within the brain, indicating a more advanced or aggressive pattern. Leptomeningeal spread occurs when cancer cells disseminate into the cerebrospinal fluid or line the surface of the brain and spinal cord, often leading to widespread neurological symptoms.
Molecular and Genetic Markers
Advances in molecular diagnostics have added a genomic dimension to staging. Key markers include:
- IDH mutation status: IDH-mutant gliomas have better prognosis.
- 1p/19q codeletion: Associated with oligodendrogliomas and a favorable treatment response.
- MGMT promoter methylation: Predicts better response to temozolomide chemotherapy.
- TERT and EGFR alterations: Common in aggressive gliomas, particularly glioblastoma.
Metastatic Brain Tumors
Metastatic brain tumors are staged based on the primary cancer site. While not staged independently, the number, size, and location of metastatic lesions affect prognosis and treatment planning. Brain metastases often come from cancers of the lung, breast, melanoma, kidney, and colon.
Causes and Risk Factors of Brain Cancer
The precise causes of brain cancer remain largely unknown, but several genetic, environmental, and lifestyle-related factors have been implicated in its development. Brain tumors, including glioblastoma and other primary brain cancers, may arise from mutations in genes that regulate cell growth, repair, and death. These mutations can lead to uncontrolled cell division and tumor formation (Louis et al., 2021).
Genetic alterations are among the most significant factors. Mutations in tumor suppressor genes (such as TP53), activation of oncogenes (like EGFR), or loss of DNA repair function (e.g., MGMT) have been linked to various types of brain cancer. Some individuals may inherit genetic syndromes such as Li-Fraumeni syndrome, Turcot syndrome, or neurofibromatosis, which increase their risk of developing brain tumors (Weller et al., 2021).
Environmental exposures, though less clearly established, are suspected to play a role. Ionizing radiation is the only well-documented environmental risk factor. Patients who have undergone radiation therapy to the head, especially during childhood, have an increased risk of developing brain tumors later in life (Ostrom et al., 2022). Age and gender also influence risk. Brain tumors can occur at any age but are more commonly diagnosed in older adults. Some tumor types, like meningiomas, are more common in women, while glioblastomas occur more frequently in men.
Immunosuppression is another factor. Individuals with compromised immune systems, including those with HIV/AIDS or those on long-term immunosuppressive therapy, may have a higher incidence of certain brain cancers, particularly primary central nervous system lymphoma. Lifestyle factors, such as cell phone use and dietary habits, have been studied extensively, but no consistent or conclusive link has been established between these and brain cancer risk.
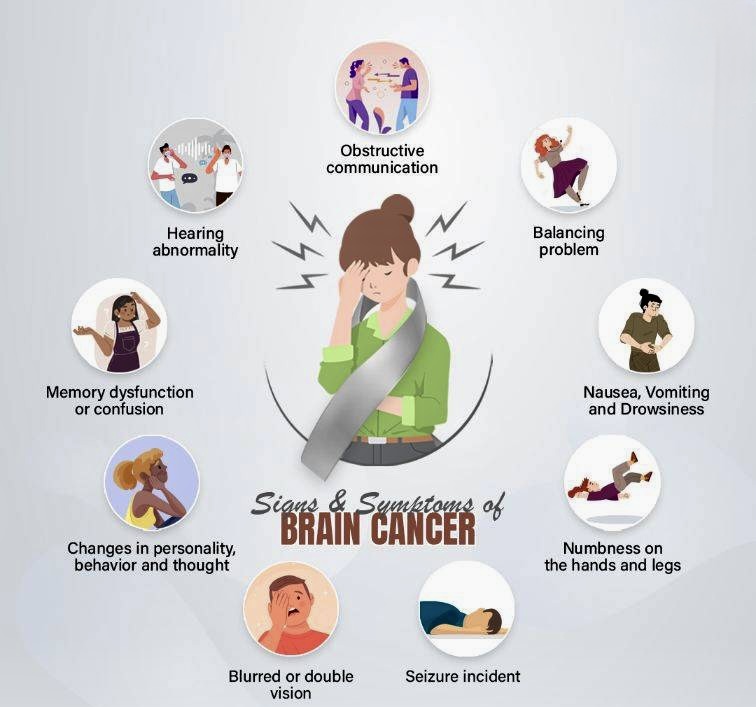
Symptoms of Brain Cancer
The symptoms of brain cancer can vary widely depending on the tumor’s type, size, and location within the brain. As the brain controls many of the body’s vital functions, any disruption caused by tumor growth can lead to a wide array of neurological or physical issues.
Headaches are among the most common early symptoms and may worsen over time or be more severe in the morning. Nausea and vomiting, especially when unrelated to other causes, may also develop due to increased intracranial pressure. Seizures can be an early sign, even in individuals with no history of epilepsy. These may present as full convulsions or subtle changes in awareness or sensation.
Cognitive and personality changes often emerge as the tumor affects specific brain regions. These can include memory loss, confusion, or sudden behavioral shifts. Speech difficulties, either in understanding or articulating words, may occur, especially with tumors in the language-dominant hemisphere. Motor skill impairments such as weakness or numbness in limbs, trouble walking, or loss of coordination are also possible.
Visual or auditory disturbances, including blurred or double vision and hearing problems, may signal tumor growth in sensory areas. In severe cases, loss of consciousness or coma can develop, especially with rapidly progressing or large tumors. Recognizing these symptoms early and seeking prompt medical evaluation is crucial for timely diagnosis and treatment.
Diagnosis of Brain Cancers
Diagnosing brain cancer involves a comprehensive clinical evaluation that includes a detailed neurologic examination, advanced neuroimaging, and tissue diagnosis through biopsy or resection. Accurate diagnosis is crucial for determining tumor type, grade, and molecular features, which guide prognosis and treatment planning.
Clinical Evaluation begins with assessment of symptoms such as persistent headaches, seizures, cognitive or personality changes, motor weakness, and visual or speech disturbances. These symptoms vary depending on the tumor’s size and location in the brain (Louis et al., 2021). Neuroimaging is the cornerstone of brain tumor diagnosis. Magnetic Resonance Imaging (MRI) with contrast is the preferred modality, offering high-resolution images of the brain and better delineation of tumor margins, edema, and enhancement patterns. Specialized MRI sequences such as diffusion-weighted imaging (DWI), perfusion MRI, and MR spectroscopy may further characterize tumor aggressiveness (Weller et al., 2021).
Computed Tomography (CT) is useful in emergency settings, particularly for detecting acute hemorrhage or calcifications, but it offers lower soft tissue contrast compared to MRI. Positron Emission Tomography (PET) using tracers like FDG or amino acid-based compounds can provide metabolic information and assist in differentiating tumor recurrence from radiation necrosis (Albert et al., 2020). Histological Confirmation is essential. Stereotactic needle biopsy is used when tumors are deep-seated or surgically inaccessible, while open surgical resection allows for both diagnosis and maximal tumor removal. Tissue samples are examined microscopically to classify the tumor and assess mitotic activity, necrosis, and cellular features.
Molecular Diagnostics now play a vital role in diagnosis. WHO CNS tumor classification incorporates molecular features such as IDH mutation status, 1p/19q codeletion, MGMT promoter methylation, and TERT promoter mutations, which influence prognosis and treatment response (Louis et al., 2021). Lumbar puncture may be considered in cases of suspected leptomeningeal spread, although it is not routinely used for parenchymal tumors.
Early and accurate diagnosis of brain cancer enables tailored treatment strategies, influences survival, and improves quality of life.
Treatment Strategies for Brain Cancer
The treatment of brain cancer is multifaceted and tailored based on tumor type, grade, location, molecular profile, patient age, and overall health status. For malignant gliomas like glioblastoma (GBM), an aggressive and multimodal approach is typically employed, while low-grade gliomas and benign tumors may require more conservative or individualized care. Treatment modalities include surgery, radiotherapy, chemotherapy, targeted therapies, and emerging interventions such as immunotherapy and tumor-treating fields (TTFields).
Surgical Resection
Surgical intervention is often the first step in managing brain tumors, especially when the tumor is accessible and causing mass effect. The primary goal is maximal safe resection—removing as much of the tumor as possible without compromising neurological function. Studies consistently show that greater extent of resection is associated with improved survival in glioblastoma and other high-grade gliomas (Lacroix et al., 2001). For low-grade gliomas, early resection may also delay progression and malignant transformation (Jakola et al., 2012).
Radiation Therapy
Postoperative radiotherapy is standard for high-grade gliomas and is frequently used in combination with chemotherapy. Fractionated external beam radiotherapy (EBRT) is typically delivered over 6 weeks. Radiotherapy plays a critical role in controlling residual disease and improving survival, especially in glioblastoma and anaplastic astrocytoma (Stupp et al., 2005). In low-grade gliomas, radiation may be deferred until signs of progression or used earlier in symptomatic cases.

Read More About Radiotherapy for Brain Tumors on Oncodaily
Chemotherapy
Temozolomide is the cornerstone chemotherapeutic agent for glioblastoma, used concurrently with radiotherapy and as adjuvant therapy for six or more cycles post-radiation. Its effectiveness is significantly influenced by the methylation status of the MGMT promoter, a predictive biomarker for treatment response (Hegi et al., 2005). In low-grade and anaplastic gliomas, chemotherapy with temozolomide or PCV (procarbazine, lomustine, vincristine) is often employed based on 1p/19q co-deletion and IDH mutation status (Buckner et al., 2016).
Tumor-Treating Fields
TTFields represent a novel, FDA-approved modality that uses alternating electric fields to disrupt mitotic spindle formation, selectively inhibiting tumor cell division. It is used as maintenance therapy in glioblastoma following chemoradiation and has shown improved progression-free and overall survival in the EF-14 trial (Stupp et al., 2017). It is non-invasive and well tolerated, though adherence to daily usage is essential for benefit.
Targeted Therapy and Molecular Approaches
Molecular profiling of brain tumors has revolutionized treatment planning. IDH mutations, 1p/19q co-deletions, and BRAF mutations are now incorporated into diagnostic algorithms and guide therapeutic choices. Bevacizumab, a VEGF inhibitor, is sometimes used in recurrent glioblastoma for symptom relief and edema control, although survival benefits are limited (Chinot et al., 2014). Ongoing research into EGFR inhibitors, FGFR inhibitors, and PI3K pathway modulators holds promise for future targeted strategies.
Immunotherapy and Vaccine-Based Therapies
Checkpoint inhibitors like nivolumab have been investigated in glioblastoma, but results to date have not demonstrated survival benefits in unselected patient populations (Reardon et al., 2020). However, interest remains in identifying subgroups that may benefit. Personalized neoantigen vaccines, dendritic cell vaccines, and CAR-T cell therapies are currently under clinical investigation and may offer future benefit as part of a combination strategy.
Read More About Immunotherapy for Brain Cancer on Oncodaily
Advances in Brain Cancer Treatment in 2025
In 2025, significant strides have been made in the treatment of brain cancer, particularly glioblastoma, through innovative therapies and technological advancements.
Pre-Surgical Use of Tumor Treating Fields
A clinical trial in Manchester, UK, is exploring the use of TTFields—a wearable device emitting low-intensity electric fields—prior to surgery for glioblastoma patients. This approach aims to disrupt tumor cell division earlier in the treatment process, potentially extending survival and altering the standard treatment timeline (The Times, 2025).
Gene Therapy with Precise Delivery Systems
Researchers at the University of Southern California have initiated a study on a novel gene therapy for glioblastoma. This therapy employs a more precise delivery system designed to target tumor cells while minimizing harm to healthy brain tissue, representing a significant step forward in gene-based treatments (Keck School of Medicine, 2025).
Optimized Chemoradiotherapy Protocols
A mathematical modeling study has proposed alternative dosing schedules for chemoradiotherapy in malignant gliomas. By adjusting treatment intervals and dosages, the model suggests potential improvements in patient survival and reductions in side effects, offering a personalized approach to therapy (Perales-Patón et al., 2025).
Sonobiopsy for Non-Invasive Diagnostics
Advancements in focused ultrasound technology have led to the development of “sonobiopsy,” a technique that temporarily opens the blood-brain barrier to allow tumor biomarkers to enter the bloodstream. This method enhances the detection of glioblastoma markers through liquid biopsy, facilitating earlier and less invasive diagnosis (Lechpammer et al., 2025).
Repurposed Drug Regimens: CUSP9v3
The CUSP9v3 protocol combines low-dose temozolomide with nine repurposed non-cytotoxic drugs to target multiple pathways involved in glioblastoma growth. Preliminary studies indicate that this multi-drug approach may inhibit tumor progression and improve treatment outcomes (Kast et al., 2024).
Rhenium-186 Nanoliposome Radiotherapy
Plus Therapeutics is developing a radiotherapeutic agent encapsulated in nanoliposomes for targeted delivery to glioblastoma tumors. This approach allows for higher radiation doses directly to the tumor while minimizing exposure to surrounding healthy tissue, potentially enhancing treatment efficacy (Plus Therapeutics, 2024).
Immunotherapy and Vaccine Combinations
Clinical trials are investigating the combination of immune checkpoint inhibitors, such as pembrolizumab, with dendritic cell vaccines in treating recurrent glioblastoma. Early results suggest that this combination may boost the immune system’s ability to recognize and attack tumor cells (UCLA Clinical Trials, 2025).
Targeted Therapy for Pediatric Brain Cancers
A collaborative effort between Emory University and QIMR Berghofer Medical Research Institute has led to the development of a targeted therapy for childhood brain cancers. The therapy focuses on the OLIG2 protein, crucial for tumor initiation and recurrence, and has shown promise in preclinical models (Emory University, 2025).
You Can Watch More on OncoDaily Youtube TV
Written by Armen Gevorgyan, MD
FAQ
What is brain cancer?
Brain cancer refers to malignant tumors originating in the brain or central nervous system. These tumors can disrupt normal brain functions and may spread within the brain or to other parts of the body.
What causes brain cancer?
The exact causes are not fully understood. However, factors such as genetic mutations, exposure to ionizing radiation, and certain inherited genetic conditions may increase the risk of developing brain cancer.
What are common symptoms of brain cancer?
Symptoms vary depending on the tumor’s location and size but can include persistent headaches, seizures, vision or hearing problems, balance issues, cognitive or personality changes, and nausea.
How is brain cancer diagnosed?
Diagnosis typically involves neurological examinations, imaging tests like MRI or CT scans, and sometimes a biopsy to determine the tumor type and grade.
What treatment options are available?
Treatment may include surgery to remove the tumor, radiation therapy, chemotherapy, targeted therapy, or a combination of these approaches, depending on the tumor’s type, location, and grade.
Is brain cancer curable?
Some brain cancers, especially when detected early and located in accessible areas, can be treated effectively. However, aggressive types like glioblastoma are challenging to cure and often require ongoing management.
What is the prognosis for brain cancer patients?
Prognosis varies widely based on tumor type, location, patient age, and overall health. For instance, glioblastoma has a median survival time of about 15 months despite treatment.
Can brain cancer spread to other parts of the body?
Primary brain cancers rarely metastasize outside the central nervous system. However, they can spread within the brain and spinal cord.
Are there risk factors for developing brain cancer?
Risk factors include exposure to high doses of ionizing radiation, family history of brain tumors, and certain genetic syndromes. Most brain cancers occur without known risk factors.
What support resources are available for patients and families?
Numerous organizations offer support, including the American Brain Tumor Association, National Brain Tumor Society, and local cancer support groups, providing information, counseling, and assistance programs.