MARIPOSA reports OS benefit with amivantamab–lazertinib vs osimertinib monotherapy!
This year, the European Lung Cancer Congress (ELCC 2025) by the European Society of Medical Oncology (ESMO) is taking place in Paris, France, from March 26 to 29.
Focused on thoracic oncology, every year the congress unites medical and radiation oncologists, thoracic surgeons, pneumologists, interventional radiologists and pathologists along a common goal – to improve multidisciplinary care for lung cancer.
The congress is co-chaired by Dr Enrico Ruffini, the Chief of the Thoracic Surgery Unit at the University of Torino, and Dr Myung-Ju Ahn, professor of Hemat-Oncology at the Department of Medicine at the Sungkyunkwan University School of Medicine, Republic of Korea.
HAPPENING NOW – attendees are actively reporting on the latest advancements presented during the congress. One of the hot topics is the MARIPOSA trial (NCT04487080), a randomized international phase 3 trial funded by Janssen Research and Development, that compared a combinational treatment approach with amivantamab–lazertinib vs osimertinib monotherapy vs lazertinib monotherapy (blindly randomized to three arms with 2:2:1 ratio) in patients with previously untreated EGFR-mutated (exon 19 deletion or L858R), locally advanced or metastatic non-small cell lung cancer (NSCLC).
Previous results published in the New England Journal of Medicine (NEJM) in 2024 showed that the trial met its primary endpoint with significantly longer median progression-free survival (PFS) in the amivantamab–lazertinib group than in the osimertinib group (23.7 vs. 16.6 months; 0.70 hazard ratio for disease progression or death; 95% confidence interval [CI], 0.58 to 0.85; P<0.001).
During ELCC25, the trial presented updated overall survival (OS) results showing benefit in the amivantamab–lazertinib vs osimertinib group.
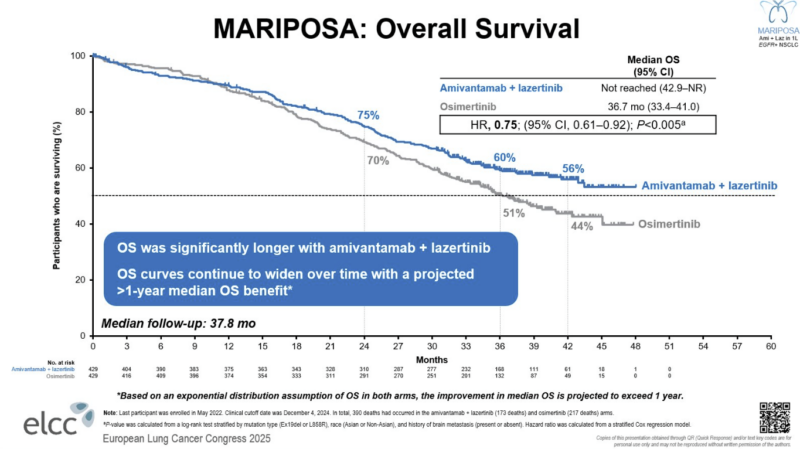
“ELCC25 MARIPOSA trial OS in 1L EGFR mut NSCLC:
Ami+Laze vs Osi:– mOS NR vs 36.7 m
– HR 0.79 for CNS progression
– median ic DoR 35.7 months
– projected OS benef > 12 months
– prophylactic anticoagulant and Steroid for iRR
Game changer in 1L
Pts with co-mutations?” – recapped Giannis Mountzios, Director of 4th Oncology Department and Clinical Trials Unit at the Henry Dunant Hospital Center, on X.
With a median OS not yet reached, the combinational arm showed minimum of 12-month benefit compared to osimertinib arm (42.9-not reached vs 36.7 months). Although, some concerns were raised by oncologists around toxicity, consistent with previous reports, and subgroup analysis. In 2024, adverse events were reported to be mainly associated with EGFR-related toxic effects, with 10% discontinuation rate for amivantamab–lazertinib and 3% for osimertinib groups.
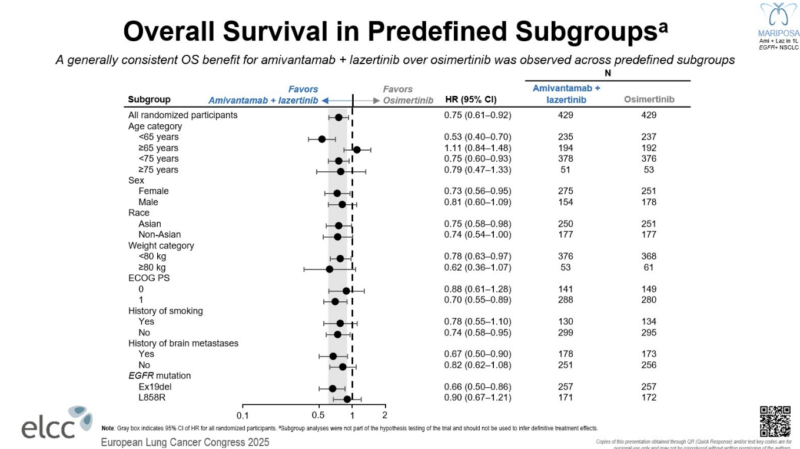
“OS in predefined subgroups from MARIPOSA
Amivantamab’s biggest challenge has been its toxicity—the degree of OS benefit won’t translate in the real world without supportive measures. Kudos to the sponsor for investing in these (IMO) practice-changing supportive measures.
Intriguing benefit/?lack of benefit of combination therapy based on age cutoff of 65″ – asks Eric Singhi, a thoracic medical oncologist at the MD Anderson Cancer Center, in his X thread.
“Debate on L1 for EGFR mut NSCLC: ‘easy osi’ then escalate to CT-ami, or use doublet upfront? Lazertinib+amivantmab likely to extend OS by ~ 1 yr vs osimertinib, but without cross over to CT-ami. Intensify only if ctEGFR not cleared after 4w of osi?” – Benjamin Besse Head of Clinical Research at Gustave Roussy, shared on X.
While ELCC25 continues in Paris, we follow the latest developments from the congress. Do not miss our daily highlights and updates from major oncology events.
“Osimertinib BEATEN!!
MARIPOSA (Ami + Lazer) shows significant OS (HR 0.75, p<0.005, 3yr 60% vs 51%) and landmark 3-yr icPFS improvement (36% vs 18%)—first combo to show OS superiority over SoC, extending OS beyond 3 years in first line EGFRm NSCLC.” – Noemi Reguart, Medical Oncologist at Hospital Clinic Barcelona, wrote on X.
Amivantamab is an EGFR-MET bispecific antibody that directs immune cells to ligand blocking, receptor degradation, and engages immune effector cells (monocytes, macrophages, and natural killer cells) with its optimized Fc domain. First-line amivantamab plus chemotherapy and second-line amivantamab monotherapy are approved for patients with EGFR exon 20 insertion–mutated advanced NSCLC.
Lazertinib is a selective third-generation EGFR-TKI, that penetrates the central nervous system (CNS) and has shown efficacy in both activating EGFR and p.Thr790Met (T790M) mutations.
Osimertinib is a third-generation EGFR TKI that targets the EGFR mutations, including the T790M mutation, which is associated with resistance to earlier treatments. Osimertinib is used in locally advanced or metastatic NSCLC and following surgery and chemotherapy.
Related to the article:
RYBREVANT plus LAZCLUZE versus osimertinib in EGFR-mutated advanced lung cancer


