The ASCO Genitourinary Cancers Symposium (ASCOGU25) is an annual event organized by the American Society of Clinical Oncology (ASCO), focusing on the latest advancements in genitourinary (GU) cancer research and treatment.
The 2025 symposium, which took place from February 13th to 15th, featured high-impact science, multidisciplinary expertise, and evidence-based practices in GU oncology.

Our team at OncoDaily has selected a few posts from Day 2 of this event that you should not miss!
“An insightful session on ‘Panning for Gold: The Role of ctDNA as a Biomarker for Bladder Cancer’! Thank you to our expert presenters for shedding light on the potential of ctDNA in advancing diagnosis and treatment.”
“Gopa Iyer with first look at LOXO435 – nice activity for FGFR3 inhibition and low AE rate. Our patients benefited from this trial!”
“Happening now at ASCOGU25! Managing toxicities of novel ADCs is one of the key challenges in bladder cancer. Great presentation by Daniel Castellano.”
“Standing room only for GCT session. Happy to see the enthusiasm to review new data and trial landscape. Time to finish the mission in testes/GCT! Great talks by Axel Heidenreich, Nabil Adra and Darren Feldman.”
“Tom Powles presents the spectacular data from EV302 with median of 2.5 years. EV-pembrolizumab continues to show maintenance of significantly improved OS and PFS compared to platinum. DoR ~ 2 years and in patients with confirmed CR, probability to remain in CR at 2 years ~74%”
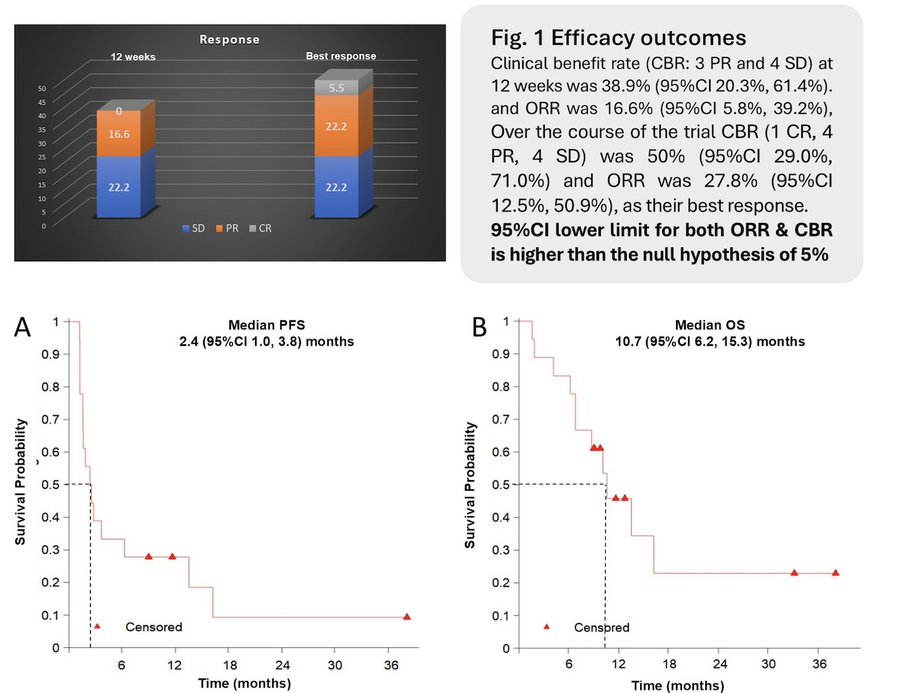
“Dr Amarnath Challapalli presented the results of EPIC-B: Phase II trial of CEMIPLIMAB as 1L in advanced penile carcinoma. The study’s primary findings were:
- up to 34 cycles Cemiplimab (PD-1 inhibitor)
- n=18, 11 UK sites
- CBR 38.9% at 12 weeks- met its primary end point
- mPFS of 2.4 months and mOS of 10.6 months
- Median 3 AEs per patient, Gr3 AEs: 6
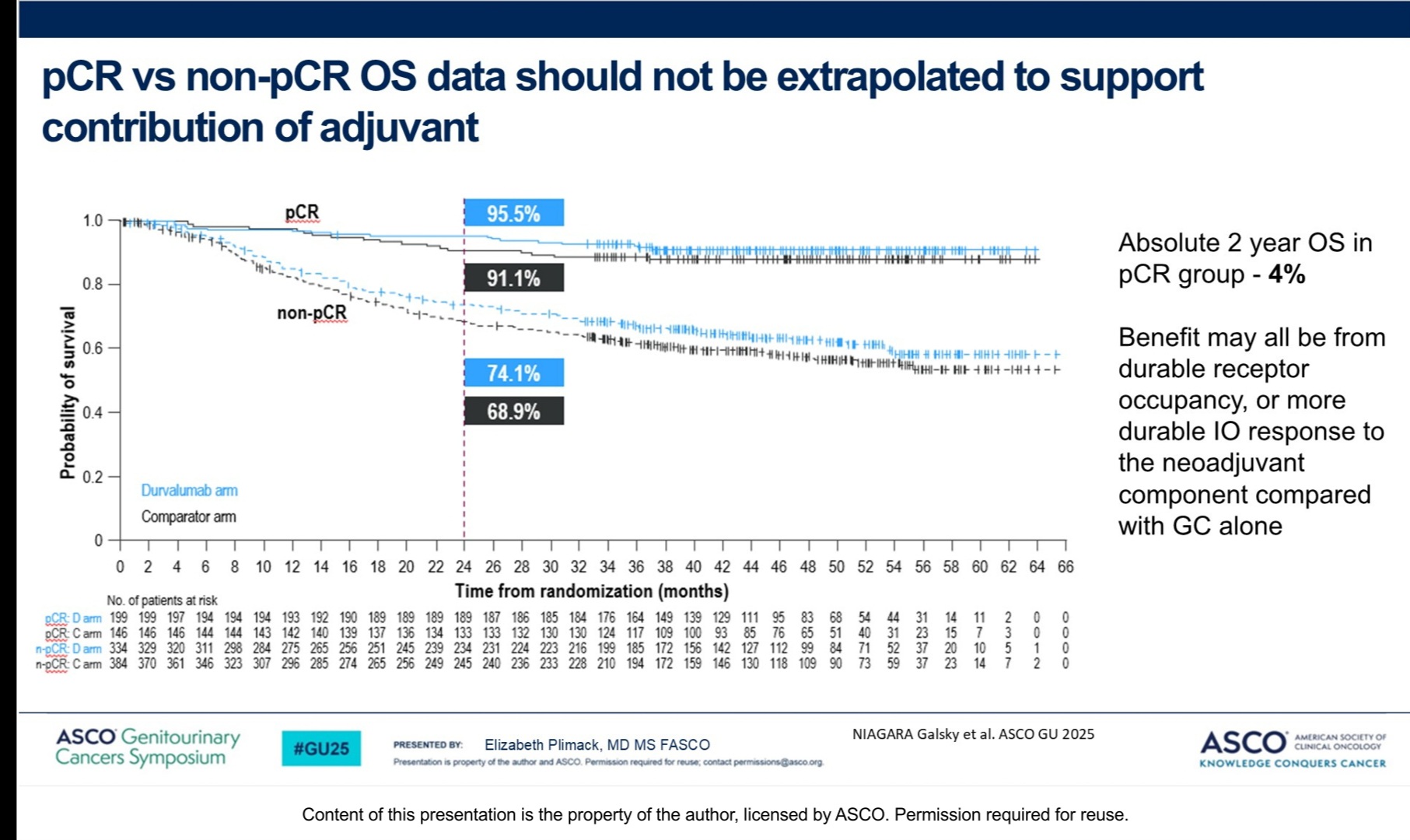
“Valid points to show pitfalls in Niagara trial . The question of benefit of neoadjuvant IO over adjuvant IO alone is unanswered. As in TNBCor NSCLC.”
“How can we apply current evidence to employ ctDNA in the optimal management of bladder cancer?”
“Fantastic success for the AFU and GETUG at American Society of Clinical Oncology (ASCO) Society GU25 with the release of the first data phase 2 trial about neoadjuvant systemic combo treatment of chemotherapy and immunotherapy before radical nephroureterectomy. • 50 patients included over 3 years in 10 French centers.
- Feasibility: confirmed.
- Tolerance: no grade 3/4 adverse events related to immunotherapy.
- Response: 13% pT0 and 50% <pT1 in the cisplatin arm.
These data are displayed in a simultaneous publication of this trial in the Journal of Clinical Oncology. Let’s keep moving forward to the Phase III.”
“Thank you Caris Life Sciences POA GU Working Group for inviting me last night to present my work on molecular alterations in intraductal prostate cancer: found to have more DNA repair alternations, high DLL3 expression, increased NEPC signature — suggestive of an aggressive subtype.”
“Durvalumab and intravesical Gemcitabine/Docetaxel shows high efficacy in BCG-unresponsive NMIBC!
- Complete Response Rate: Overall: 89%, CIS: 87% , Papillary tumors: 92%
- Safety: Mostly low-grade AEs”
“Thanks for the great catch-up, Dr. Christopher Wee from Cleveland Clinic at GU25! Your support during my medical oncology away rotation and the letter of recommendation for my heme/onc fellowship training were invaluable. Couldn’t have done it without your help!”

“Bladder Cancer Rapid Oral Abstracts at ASCOGU25
Dr. Alina Basnet presenting Abs666: Genomic Landscape of Young-Onset Bladder Cancer
Study Details:
- N = 9,411 patients with CAUBC
- 291 (3.1%) were younger than 50 years
- Comprehensive Genomic Profiling (CGP) conducted
Key Findings:
- Genomic Alterations (GA) and Tumor Characteristics:
- Higher GA/tumor burden in CAUBC >50: 8.0 vs 7.0 (p<.0001)
- Higher TMB >10 in CAUBC >50: 35.1% vs 28.5% (P=.048)
- Higher APOBEC signature in CAUBC >50: 32.5% vs 25.8% (p=.041)
- MSI-high status is extremely uncommon: 0.3%-0.8% (HR, 0.51, 0.43-0.61)
Genomic Alterations More Common in CAUBC >50:
- KMD6A: 26.6% vs 14.8% (p=.0001)
- ERBB2: 18.4% vs 16.8% (p=.85)
- ERBB3: 6.3% vs 6.5% (p=.91)
- MTAP: 25.0% vs 20.3% (p=.27)
- TERT: 78.0% vs 68.4% (p=.005)
Genomic Alterations More Common in CAUBC <50:
- HRAS: 4.8% vs 2.1% (p=.11)
- CCND1: 16.2% vs 13.1% (p=.35)
Genomic Alterations with Similar Prevalence in CAUBC <50 & >50:
- FGFR1: 4.1% vs 5.2% (p=.71)
- FGFR3: 14.1% vs 17.9% (p=.31)
- PIK3CA: 20.3% vs 21.3% (p=.91)
- PTEN: 6.5% vs 4.5% (p=.31)
Clinical Outcomes:
- Overall Response Rate (ORR): 67.5% vs 44.2%
- Duration of Response (DOR): 23.3 months vs 7.0 months
- Complete Response (CR): 30.4% vs 14.5%
- Duration of CR: Not Reached vs 14.5 months
Clinical Implications:
- CAUBC in patients under 50 years has a unique biomarker and GA distribution pattern.
- Can GA guide new therapies for young-onset bladder cancer?”
“BCG-unresponsive disease for NMIBC- what would you do next?
Majority of room would proceed to cystectomy…but perhaps consider chemoRT? Dr. Brian Baumann takes us through the data Ps consider opening and enrolling to NRG GU014 (opens in March!)”
“Awesome Research To Practice Symposium regarding management of RCC by panelists – Rana McKay, Tian Zhang and Thomas Hutson and moderator Sumanta Pal, review of IO and TKI in ccRCC, optimal treatment of relapsed/refractory RCC, and role of HIF-2a inhibitors in VHL-associated RCC.”