ASCO GI 2026, taking place January 8–10 at Moscone West in San Francisco, California, with online access, is expected to deliver practice-relevant updates across gastrointestinal (GI) cancers, including gastric, esophageal, colorectal, pancreatic, and hepatocellular carcinoma (HCC). The program will feature biomarker-defined treatment strategies, novel antibody platforms, alternative chemotherapy backbones, perioperative optimization approaches, and long-term follow-up analyses across multiple disease settings.
This overview highlights selected GI cancer studies scheduled for presentation at ASCO GI 2026, with potential to inform clinical practice and contribute to the evolution of future standards of care.
Phase II ILUSTRO Trial: Zolbetuximab + mFOLFOX6 + Nivolumab in CLDN18.2-Positive Gastric/GEJ Adenocarcinoma
Abstract: LBA284
Presenter: Kohei Shitara (Kashiwa, Japan)
Trial Type: Phase II
Session: Oral Abstract Session
ILUSTRO is a phase II, multicohort study evaluating zolbetuximab, a CLDN18.2-targeting monoclonal antibody, in combination with chemotherapy and immunotherapy in CLDN18.2-positive gastric and gastroesophageal junction adenocarcinoma. At ASCO GI 2026, the study presents a first-line assessment of zolbetuximab combined with mFOLFOX6 and nivolumab in patients with locally advanced unresectable or metastatic disease.
This regimen explores whether integrating tumor-selective antibody targeting with PD-1 blockade and platinum-based chemotherapy can refine first-line strategies in a biomarker-defined population.

You can also read about Zolbetuximab (Vyloy) on OncoDaily.
Zanidatamab + chemotherapy (CT) ± tislelizumab for first-line (1L) HER2-positive (HER2+) locally advanced, unresectable, or metastatic gastroesophageal adenocarcinoma (mGEA): Primary analysis from HERIZON-GEA-01.
Abstract: LBA285
Presenter: Elena Elimova (Toronto, Canada)
Trial Type: Phase III, Randomized
Session: Oral Abstract Session
HERIZON-GEA-01 is a global, randomized phase III trial evaluating zanidatamab plus chemotherapy, with or without tislelizumab, versus trastuzumab plus chemotherapy as first-line treatment for HER2-positive locally advanced unresectable or metastatic gastroesophageal adenocarcinoma. Zanidatamab is a bispecific, biparatopic anti-HER2 antibody designed to enhance HER2 clustering and immune engagement.
This primary analysis assesses the role of zanidatamab-based regimens compared with the current trastuzumab-based standard, with implications for the future first-line HER2-targeted backbone in advanced disease.

Read more about HERIZON-GEA-01 on OncoDaily.
Liposomal irinotecan, carboplatin or oxaliplatin (LyRICX) with or without nivolumab in the first-line treatment of metastatic or irresectable esophagogastric adenocarcinoma: A randomized phase 2 study.
Abstract: LBA287
Presenter: Denice Kamp (Utrecht, Netherlands)
Trial Type: Phase II, Randomized
Session: Rapid Oral Abstract Session
LyRICX is a randomized phase II study evaluating liposomal irinotecan-, carboplatin-, or oxaliplatin-based chemotherapy, with or without nivolumab, as first-line treatment for metastatic or irresectable esophagogastric adenocarcinoma. The trial compares progression-free survival and treatment-related neurotoxicity across different chemotherapy backbones, with nivolumab use guided by PD-L1 status.
The study addresses whether non-oxaliplatin strategies can optimize the balance between efficacy and tolerability in the first-line setting.
Read more about Opdivo (Nivolumab) on OncoDaily.
Nivolumab plus ipilimumab vs lenvatinib or sorafenib as first-line treatment for unresectable hepatocellular carcinoma (HCC): 4-year follow-up of CheckMate 9DW.
Abstract: LBA479
Presenter: Peter Robert Galle (Mainz, Germany)
Trial Type: Phase III, Randomized
Session: Rapid Oral Abstract Session
This randomized phase III study compares nivolumab plus ipilimumab with standard first-line therapy using lenvatinib or sorafenib in patients with unresectable hepatocellular carcinoma. The primary endpoint is overall survival, with secondary endpoints including objective response rate, duration of response, and time to symptom deterioration. This presentation reports the 4-year follow-up of the CheckMate 9DW trial.
Read more Sorafenib (Nexavar) on OncoDaily.
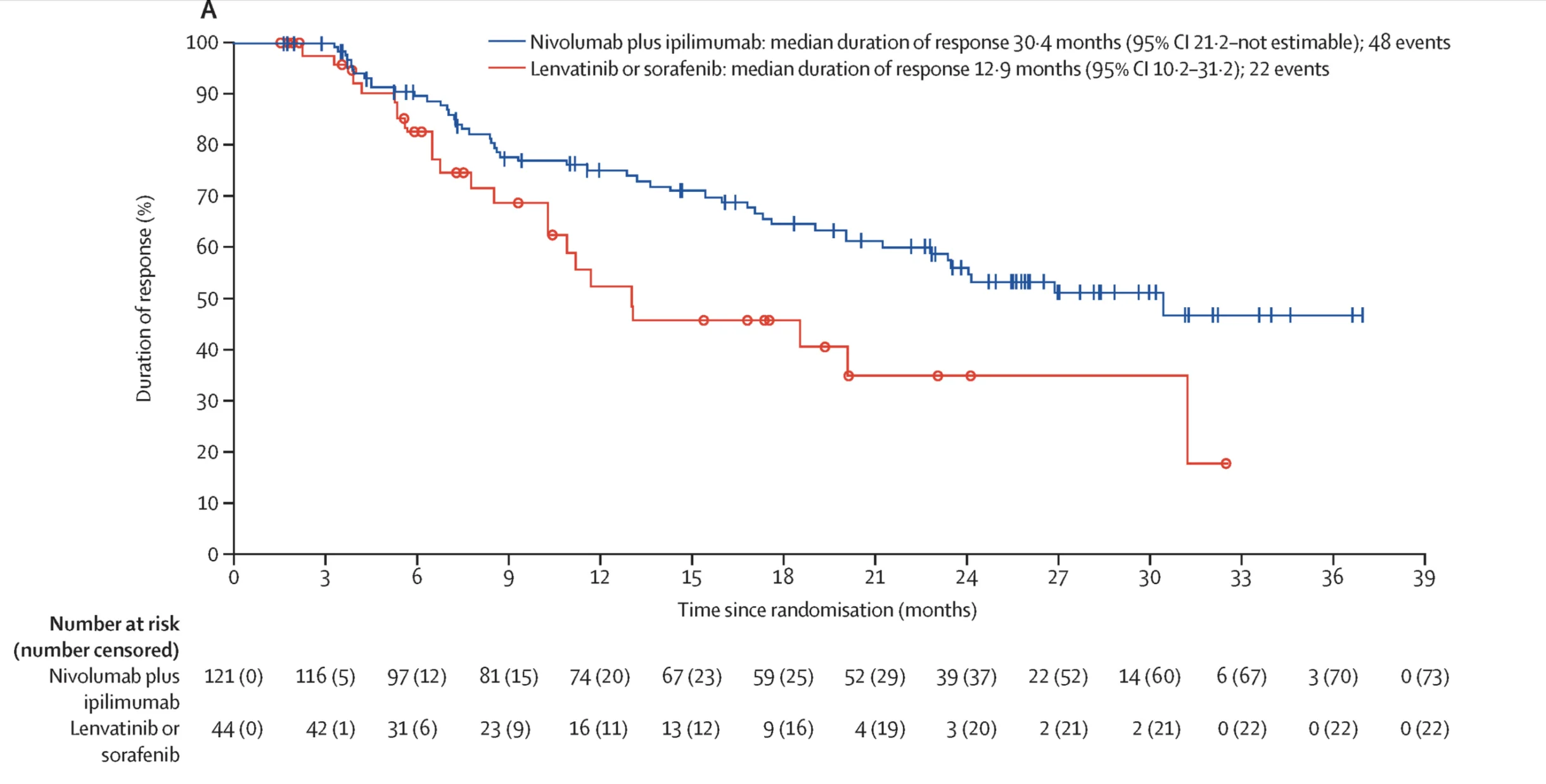
Previously, results from the phase III CheckMate 9DW trial, published in The Lancet in 2025, demonstrated a significant overall survival benefit with nivolumab plus ipilimumab compared with lenvatinib or sorafenib in previously untreated unresectable hepatocellular carcinoma.
Key findings included:
- Median overall survival of 23.7 months with nivolumab plus ipilimumab versus 20.6 months with lenvatinib or sorafenib
- Higher long-term survival rates with dual immunotherapy, with 38% vs 24% alive at 36 months
- A manageable safety profile, with comparable rates of grade 3–4 treatment-related adverse events between groups
The current presentation provides extended follow-up, offering further insight into the durability of benefit with this first-line immunotherapy strategy.
Associations between ECOG performance status (PS) and patient-reported outcomes (PROs) in patients with gastric or gastroesophageal junction (G/CEJC) adenocarcinoma: Results from the RATIONALE-305 trial.
Abstract: 288
Presenter: Ken Kato (Tokyo, Japan)
Trial Type: Exploratory Analysis (Phase III trial dataset)
Session: Rapid Oral Abstract Session
This analysis from the RATIONALE-305 trial examines the relationship between baseline ECOG performance status and patient-reported outcomes in patients with gastric or gastroesophageal junction adenocarcinoma. Validated instruments were used to assess global health status, functional domains, and disease-specific symptoms at baseline.
The study explores whether clinician-reported performance status adequately reflects patient-reported symptom burden and quality of life at treatment initiation.
BREAKWATER: Primary analysis of first-line (1L) encorafenib + cetuximab (EC) + FOLFIRI in BRAF V600E-mutant metastatic colorectal cancer (mCRC)
Abstract: 13
Presenter: Scott Kopetz (Houston, United States)
Trial Type: Phase III, Randomized
Session: Oral Abstract Session
BREAKWATER evaluates encorafenib plus cetuximab in combination with FOLFIRI as first-line treatment for patients with BRAF V600E-mutant metastatic colorectal cancer. The study builds on earlier BREAKWATER cohorts supporting combined BRAF and EGFR inhibition with chemotherapy in this molecularly defined population.
Previously, results from the phase III BREAKWATER study presented at the ESMO Congress 2025 in Berlin established encorafenib plus cetuximab with mFOLFOX6 as a new first-line standard for BRAF V600E–mutant metastatic colorectal cancer. In that analysis, objective response rates reached 63–75% with EC + mFOLFOX6, compared with 40–49% with EC alone and ~40% with control chemotherapy, across both high and low baseline ctDNA burden subgroups.
Overall survival favored EC + mFOLFOX6, with hazard ratios of 0.39–0.50 versus control, and early ctDNA clearance at cycle 2 day 15 occurred in 67% of patients, correlating with markedly improved survival (OS HR 0.30 for ctDNA cleared vs detectable). In addition, the combination was associated with lower rates of acquired MAPK pathway resistance mutations compared with EC monotherapy.
Building on these data, the current ASCO GI 2026 presentation reports the primary analysis of encorafenib plus cetuximab with FOLFIRI.
You can also read about BREAKWATER Study Results at ESMO 2025: ctDNA Clearance and Resistance Evolution in BRAF V600E–Mutant Colorectal Cancer on OncoDaily.
Preliminary phase 1 results of INCB161734, a novel oral Kirsten rat sarcoma (KRAS) G12D inhibitor, as monotherapy or in combination with chemotherapy for advanced/metastatic pancreatic duct adenocarcinoma (PDAC)
Abstract: 654
Presenter: Zev A. Wainberg (Los Angeles, United States)
Trial Type: Phase I
Session: Oral Abstract Session
INCB161734 is a novel, selective oral small-molecule inhibitor targeting KRAS G12D, the most prevalent KRAS alteration in pancreatic ductal adenocarcinoma. This first-in-human phase I study evaluates INCB161734 as monotherapy and in combination with standard chemotherapy regimens, including gemcitabine plus nab-paclitaxel and mFOLFIRINOX, in patients with advanced or metastatic KRAS G12D-mutant disease.
The trial focuses on safety, pharmacokinetics, and preliminary antitumor activity, with integrated translational analyses such as longitudinal ctDNA monitoring.
Adjuvant pembrolizumab for participants with hepatocellular carcinoma and complete radiologic response after surgical resection or local ablation: The phase 3 keynote-937 study
Abstract: 477
Presenter: Stephen Lam Chan (Hong Kong, China)
Trial Type: Phase III, Randomized, Double-Blind
Session: Oral Abstract Session
KEYNOTE-937 is a global phase III randomized, double-blind trial evaluating pembrolizumab versus placebo as adjuvant therapy in patients with hepatocellular carcinoma who achieved a complete radiologic response following surgical resection or local ablation.
The study addresses the unmet need for effective adjuvant strategies in HCC and evaluates whether PD-1 blockade can reduce recurrence risk and improve long-term outcomes following curative-intent treatment.
CRITICS-II: A multicenter randomized phase II trial of neo-adjuvant chemotherapy followed by surgery versus neo-adjuvant chemotherapy and subsequent chemo-radiotherapy followed by surgery versus neo-adjuvant chemoradiotherapy followed by surgery in resectable gastric cancer.
Abstract: 283
Presenter: Marcel Verheij (Netherlands)
Trial Type: Phase II, Multicenter, Randomized
Session: Oral Abstract Session
CRITICS-II is a multicenter, randomized phase II trial conducted by the Dutch Upper GI Cancer Group, designed to evaluate and compare three preoperative treatment strategies for patients with resectable, non-metastatic gastric adenocarcinoma. The study specifically explores the optimal sequencing and intensity of neoadjuvant therapy while omitting postoperative treatment, aiming to improve treatment compliance and surgical outcomes.
Patients with clinical stage IB–IIIC gastric cancer were randomized to receive one of three neoadjuvant approaches: chemotherapy alone, chemotherapy followed by chemoradiotherapy, or chemoradiotherapy alone, each followed by surgery. The trial was designed to assess feasibility, safety, and activity of these strategies, with the goal of identifying the most suitable regimen for further phase III evaluation in the context of evolving perioperative treatment paradigms.
In addition to clinical and surgical endpoints, CRITICS-II incorporates translational research to explore predictive and prognostic biomarkers, supporting future treatment personalization in resectable gastric cancer.
Adjuvant oxaliplatin with S-1 (SOX) versus S-1 in patients with stage II-III gastric or gastro-oesophageal junction adenocarcinoma (CAPITAL): A randomised, open-label, phase 3 trial.
Abstract: 289
Presenter: Run-Cong Nie (Guangzhou, China)
Trial Type: Phase III, Multicenter, Randomized, Open-Label
Session: Rapid Oral Abstract Session
CAPITAL is a multicenter phase III trial evaluating whether adding oxaliplatin to standard S-1 adjuvant therapy improves postoperative management of stage II–III gastric or gastro-oesophageal junction adenocarcinoma following D2 gastrectomy. Patients were randomized to receive either S-1 alone or a sequential oxaliplatin plus S-1 regimen.
The study addresses whether intensifying adjuvant treatment beyond S-1 monotherapy can further reduce recurrence risk while maintaining acceptable safety in this curative-intent setting.
Learn more about the 2026 ASCO Gastrointestinal Cancers Symposium.


