
EMBER-3 trial Results: Patient-Reported Outcomes with Imlunestrant ± Abemaciclib vs Standard Endocrine Therapy in ER+/HER2− Advanced Breast Cancer
At the ASCO Congress 2025, Dr. Giuseppe Curigliano and colleagues presented patient-reported outcomes (PRO) from the Phase III EMBER-3 trial, evaluating the novel oral selective estrogen receptor degrader (SERD) imlunestrant (imlu) in patients with estrogen receptor–positive, HER2-negative (ER+/HER2−) advanced breast cancer (ABC) who had progressed on aromatase inhibitor therapy. The trial demonstrated that imlu, both alone and in combination with abemaciclib, not only improved progression-free survival (PFS) in patients with ESR1 mutations (ESR1m) but also preserved or improved quality-of-life (QoL) scores compared to standard-of-care endocrine therapy.
What Is the EMBER-3 Trial?
EMBER-3 is a global, randomized, Phase III trial evaluating imlunestrant versus standard-of-care (SOC) therapies—either fulvestrant or exemestane—in patients with ER+/HER2− ABC. The study also included a comparison between imlu+abemaciclib (abema) and imlu alone. The trial enrolled a biologically heterogeneous population, with a subset of patients carrying ESR1 mutations, a known mechanism of endocrine resistance.
Key investigators included Drs. Joyce O’Shaughnessy (Texas Oncology), François Bidard (Institut Curie), Sung-Bae Kim (Asan Medical Center), and Nadia Harbeck (LMU Munich), alongside collaborators from 18 institutions across Europe, Asia, Australia, and the Americas.
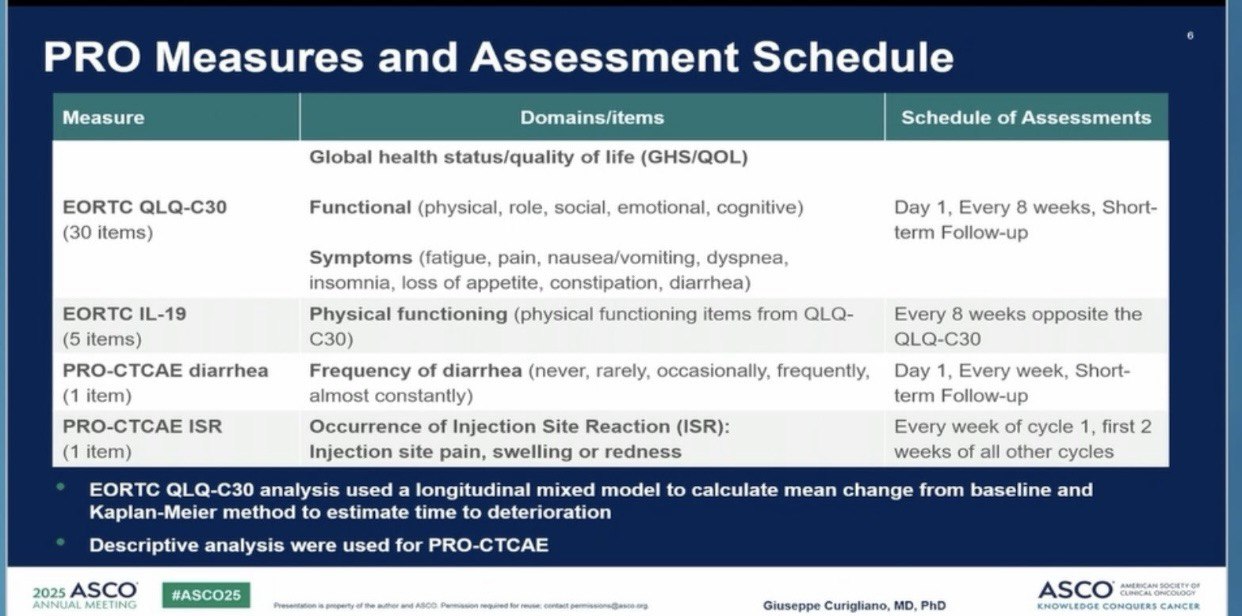
Trial Design and Patient-Reported Outcomes
Patients completed the EORTC QLQ-C30 at baseline and every 8 weeks, while weekly PRO-CTCAE data captured injection site reactions (ISR) and diarrhea frequency. The primary endpoint of the current analysis was mean change from baseline in global health status/quality of life (GHS/QOL) and physical function (PF) scores, analyzed using a longitudinal mixed model.
Key Findings from EMBER-3: Patient-Centered Outcomes with Imlunestrant
In the EMBER-3 trial, imlunestrant demonstrated clear benefits in patient-reported outcomes, particularly for individuals with ESR1 mutations—a subgroup often resistant to traditional endocrine therapies.
Benefits for ESR1-Mutant Patients
For patients carrying ESR1 mutations, imlunestrant monotherapy offered a marked improvement in both quality of life and physical function. On average, patients reported a 9.9-point improvement in global health status and quality of life (GHS/QOL), and a 6.2-point increase in physical functioning, compared to those receiving standard endocrine therapies like fulvestrant or exemestane. These results not only highlight imlu’s clinical effectiveness but also its ability to enhance day-to-day well-being, offering a dual advantage that aligns with its previously demonstrated progression-free survival benefit.
Outcomes in the Overall Population
When looking at the broader population—including patients without ESR1 mutations—the differences between imlu and standard therapies were less pronounced but still notable. Quality-of-life scores remained comparable between groups, with a slight edge for imlu (+0.5 points). More importantly, physical functioning was better preserved with imlu, showing a +2.5-point average improvement, suggesting that patients on imlu may retain their functional independence and daily activity levels more effectively than those on traditional endocrine treatments.
Does Adding Abemaciclib Help?
The study also explored whether combining abemaciclib, a CDK4/6 inhibitor, with imlu would provide additional benefits. In terms of patient-reported outcomes, the combination did not significantly improve quality of life or physical function compared to imlu alone. The global health score was virtually the same (+0.8 points), while physical function showed a minor decline (−2.2 points), which was not considered clinically meaningful. These findings suggest that while the combination remains tolerable, it may not enhance patients’ quality of life beyond what is achieved with imlu monotherapy.
Side Effects and Patient Experience
Two areas stood out in terms of side effects: diarrhea and injection site reactions (ISRs).
-
Diarrhea was considerably more common in the abemaciclib combination group, with 22% of patients reporting it as frequent or constant. By contrast, only 3% of those on imlu alone and 2% of those on standard therapy experienced diarrhea at similar levels.
-
ISRs were frequently reported among patients receiving fulvestrant, with 72% experiencing issues such as pain, swelling, or redness at the injection site. Notably, these side effects were more accurately captured by patient-reported tools (PRO-CTCAE) than by clinician documentation, indicating the importance of incorporating direct patient feedback into toxicity monitoring.
What This Means for Patients
The EMBER-3 trial adds compelling patient-centered data to the growing evidence base for imlunestrant in endocrine-resistant ER+/HER2− ABC. Patients with ESR1 mutations, a difficult-to-treat population, experienced both clinical efficacy and improved quality of life with oral imlu therapy compared to existing injectable SERDs.
Additionally, the low burden of gastrointestinal toxicity and absence of injection-related adverse events with imlu monotherapy reinforce its favorable tolerability. As the oncology field continues moving toward personalized, biomarker-driven treatment, these results support the role of oral SERDs like imlu in improving outcomes beyond traditional measures of tumor shrinkage.
What People Are Saying About the EMBER-3 Trial
Caterina Sposetti MD, Research Fellow of Dana-Farber’s Breast Oncology Center shared on X
“Brilliant presentation of EMBER-3 trial PROs: only by bridging the gap between patient experience and clinician-reported adverse events can we provide the best care to our patients!”
Amylou Dueck, PhD Vice chair, Quantitative Health Sciences, Mayo Clinic shared on X
“Kudos to ASCO25 for including PROs in the big oral sessions! Nice presentation of EORTC and PRO-CTCAE data in EMBER-3 trial (imlunestrant vs SOC for ER+ advanced breast cancer) by Giuseppe Curigliano. Injection site reaction item of PRO-CTCAE featured prominently here!”

You Can Also Read About KANDLELIT-001 Trial Demonstrates Promising Activity of MK-1084 in KRAS G12C–Mutant Colorectal Cancer at ASCO 2025
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
