
DESTINY-Breast06 Trial: T-DXd Confirms Superiority Over Chemotherapy in HR+/HER2-Low and Ultralow Metastatic Breast Cancer
DESTINY-Breast06 trial data presented at the ESMO Breast Cancer 2025 Congress in Munich, May 14, 2025 further solidify trastuzumab deruxtecan (T-DXd) as a preferred post-endocrine therapy option in patients with hormone receptor-positive (HR+), HER2-low and HER2-ultralow metastatic breast cancer, marking a pivotal advance in the HER2-targeted therapeutic continuum.
“In this analysis of DESTINY-Breast06, T-DXd demonstrated a clinically meaningful efficacy benefit over both treatment of physician’s choice subgroups, with improved PFS, ORR, DOR, and PFS2,” said Dr. Carlos H. Barrios, lead study author and president of LACOG (Latin American Cooperative Oncology Group). Barrios emphasized that the superiority of T-DXd extended consistently across all major clinical endpoints.

Image Taken from the DESTINY-Breast06 Phase III Trial Presentation at ESMO Breast Cancer 2025
Trial Design and Clinical Context
DESTINY-Breast06 was a multicenter, open-label, phase 3 trial that enrolled 866 patients with HR+ metastatic breast cancer characterized as HER2-low (IHC 1+ or 2+/ISH−) or HER2-ultralow (IHC 0 with faint membrane staining). These patients were chemotherapy-naïve in the metastatic setting and had received:
- At least two lines of endocrine therapy (with or without targeted therapy), or
- One line of endocrine therapy and progression within six months of starting endocrine + CDK4/6 inhibitor treatment, or
- Recurrence within 24 months of adjuvant endocrine therapy.
Patients were randomized 1:1 to receive T-DXd (5.4 mg/kg IV every 3 weeks) or treatment of physician’s choice (TPC) — either capecitabine (59.8%) or a taxane (40.2%). The primary endpoint was progression-free survival (PFS) in the overall intention-to-treat (ITT) population, with secondary endpoints including overall response rate (ORR), duration of response (DOR), PFS2 (progression-free survival on next-line therapy), and safety.
Efficacy Outcomes: Subgroup and Total Population Insights
The primary analysis had already shown a 5.1-month PFS improvement in the ITT population favouring T-DXd: Median PFS:
- T-DXd: 13.3 months
- TPC: 8.2 months
- HR: 0.64 (95% CI: 0.54–0.76; P < .001)
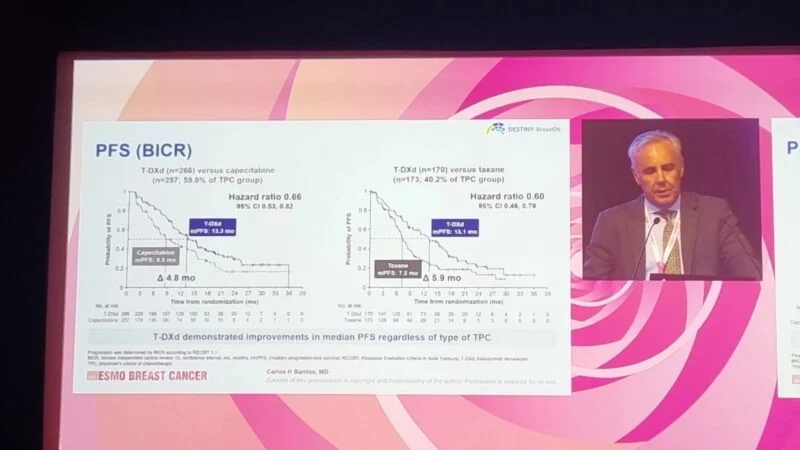
Image Taken from the DESTINY-Breast06 Phase III Trial Presentation at ESMO Breast Cancer 2025
At ESMO 2025, further subgroup analyses were presented, showing that T-DXd consistently outperformed both chemotherapy agents:
Objective Response Rate (ORR)
- T-DXd vs Capecitabine: 57.9% vs 30.7%
- T-DXd vs Taxane: 56.5% vs 31.8%
Duration of Response (DOR)
- T-DXd vs Capecitabine: 14.6 months vs 11.4 months
- T-DXd vs Taxane: 13.7 months vs 6.2 months
PFS2 (Progression-Free Survival after Next-Line Therapy)
- T-DXd vs Capecitabine: 22.6 vs 16.4 months (HR: 0.62; 95% CI: 0.49–0.78)
- T-DXd vs Taxane: 18.2 vs 13.6 months (HR: 0.61; 95% CI: 0.47–0.80)
These results highlight not only the initial benefit of T-DXd but also its potential to extend disease control beyond first progression, a key consideration in HR+ metastatic disease.
Regulatory Impact
Based on earlier DESTINY-Breast06 findings, the U.S. FDA approved T-DXd in January 2025 for the treatment of unresectable or metastatic HR+/HER2-low or ultralow breast cancer following endocrine resistance. This marked the first regulatory recognition of HER2-ultralow as a therapeutic target. At the same time, the Ventana PATHWAY anti-HER2 (4B5) assay was approved as a companion diagnostic for detecting HER2-ultralow tumors.
Safety Profile
Adverse events were consistent with previous reports. T-DXd showed a manageable safety profile, with certain toxicities more frequent compared to chemotherapy but generally offset by longer treatment durations.
Any-grade TRAEs (T-DXd vs Capecitabine):
- Nausea: 68.7% vs 28.1%
- Fatigue: 48.7% vs 34.5%
- Neutropenia: 35.1% vs 17.7%
- Vomiting: 25.3% vs 10.8%
- Palmar-plantar erythrodysesthesia: 0.4% vs 53.4%
Image Taken from the DESTINY-Breast06 Phase III Trial Presentation at ESMO Breast Cancer 2025
Any-grade TRAEs (T-DXd vs Taxane):
- Nausea: 61.5% vs 16.7%
- Fatigue: 43.8% vs 33.9%
- Neutropenia: 41.4% vs 42.3%
- Vomiting: 30.2% vs 7.1%
- Peripheral sensory neuropathy: 1.8% vs 20.8%
Barrios highlighted that neutropenia was the most common grade ≥3 TRAE with both T-DXd and taxanes, while hand-foot syndrome was predominant with capecitabine.
“When adjusted for treatment duration, the overall safety profile of T-DXd was generally similar to or better than that of capecitabine or taxane,” Barrios concluded.
Expert Perspective
“When adjusted for treatment duration, the overall safety profile of T-DXd was generally similar to or better than that of capecitabine or taxane,” Barrios stated. “These findings further support T-DXd as an effective treatment option in HR+/HER2-low or ultralow metastatic breast cancer after at least one line of endocrine therapy.”
He concluded, “With improvements in PFS, response rate, and durability—and a predictable, manageable toxicity profile—T-DXd is redefining how we approach HER2-expressing breast cancers, even at the lowest levels.”
Discussion
The DESTINY-Breast06 trial represents a turning point in the management of hormone receptor-positive, HER2-low and HER2-ultralow metastatic breast cancer. The results presented at ESMO Breast Cancer 2025 not only confirm the statistically significant and clinically meaningful benefit of T-DXd over traditional chemotherapy but also validate HER2 expression as a therapeutic continuum, rather than a binary biomarker.
Historically, patients with HER2-low or ultralow tumors were considered HER2-negative and excluded from HER2-targeted therapies. This study challenges that paradigm. The consistent superiority of T-DXd in terms of PFS, ORR, DOR, and PFS2 across both HER2-low and ultralow subgroups confirms that even minimal HER2 expression is sufficient for T-DXd activity. Importantly, the benefits were observed regardless of the comparator—whether capecitabine or taxane—highlighting the robustness of the drug’s efficacy.
Safety remains an essential consideration in metastatic settings, where long-term disease control and quality of life are equally important. T-DXd’s safety profile was consistent and manageable, with a tolerability profile that compares favorably to standard chemotherapy when adjusted for duration of exposure. Notably, neurotoxicity and hand-foot syndrome—common limiting factors for taxanes and capecitabine, respectively—were less prominent with T-DXd.
The FDA’s decision to approve T-DXd for HER2-ultralow breast cancer, alongside the expanded use of companion diagnostics, marks a regulatory milestone. This trial has helped define HER2-ultralow as a clinically actionable subgroup, expanding treatment options for thousands of patients worldwide.
Looking forward, DESTINY-Breast06 raises critical questions about redefining HER2 classification in clinical practice. Should HER2-ultralow be recognized as a distinct therapeutic category? Should all metastatic HR+ patients undergo advanced HER2 assessment? These questions, while not yet fully answered, are now front and center in the evolving landscape of targeted therapy.
In conclusion, DESTINY-Breast06 sets a new standard for post-endocrine therapy in HR+/HER2-low and ultralow disease and paves the way for broader adoption of antibody-drug conjugates in previously under-addressed populations. The trial’s findings highlight the power of molecular precision in reshaping metastatic breast cancer care.
Also Read About EMBER-3 Trial Updates on Oncodaily
Disclosures
Dr. Barrios reported consulting fees and honoraria from AstraZeneca, Daiichi Sankyo, Lilly, MSD, Novartis, Pfizer, Roche/Genentech, and others. He also holds equity in MedSIR and Tummi.
You Can Watch More on OncoDaily Youtube TV
Written by Armen Gevorgyan, MD
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
