Presented by Dr. Cristina Saura Manich (Barcelona, Spain) at the ESMO Breast Cancer Annual Congress 2025, new data from the EMBER-3 trial spotlight a promising endocrine-based treatment strategy for patients with estrogen receptor-positive, HER2-negative (ER+/HER2-) advanced breast cancer who have progressed after aromatase inhibitors, with or without prior CDK4/6 inhibitor therapy.
What Drug is Imlunestrant?
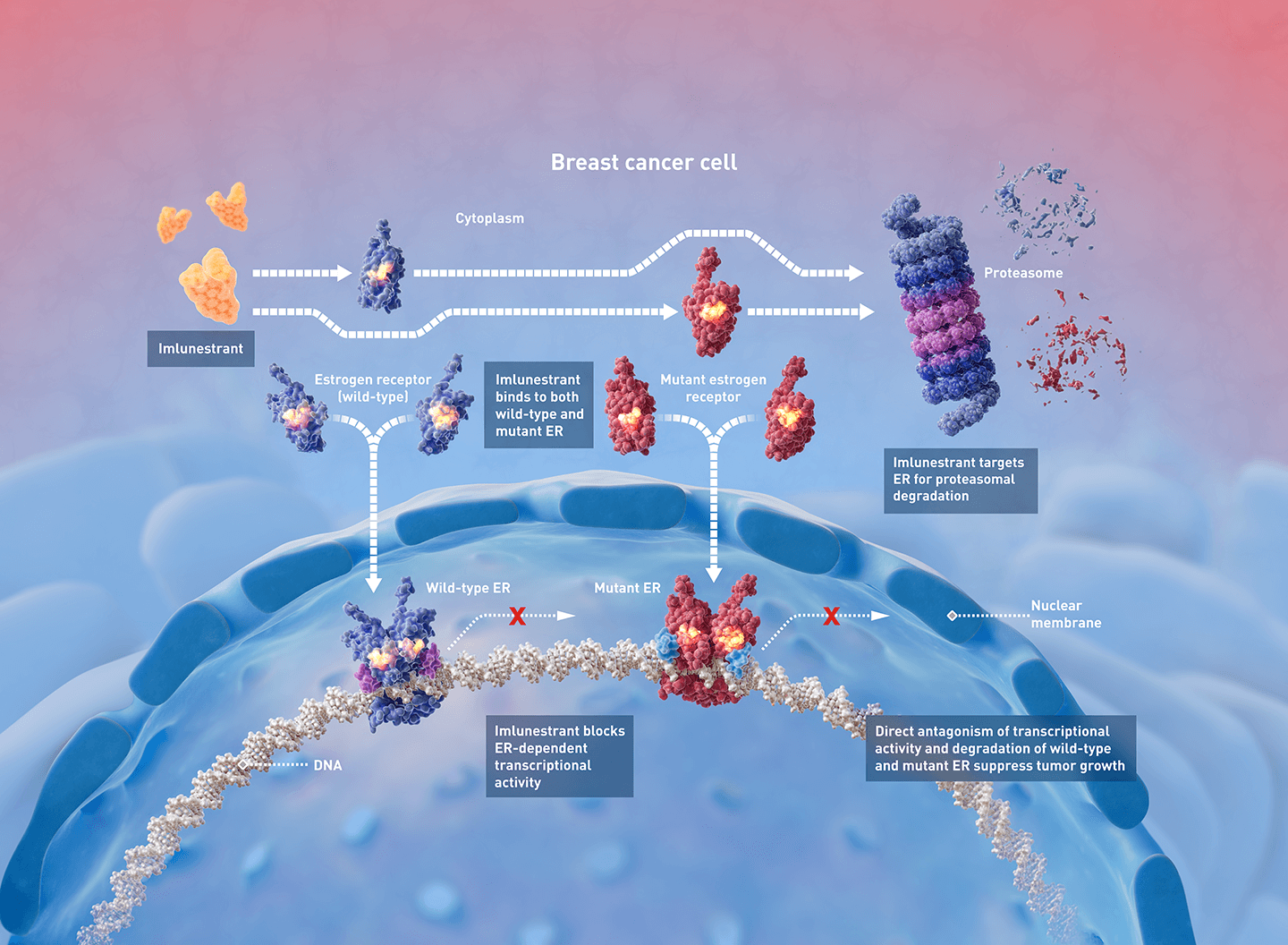
Imlunestrant is a next-generation oral selective estrogen receptor degrader (SERD) with brain-penetrant properties, designed to overcome resistance mechanisms, including ESR1 mutations. The EMBER-3 trial previously demonstrated that imlu significantly improved progression-free survival (PFS) compared to standard endocrine therapy (fulvestrant or exemestane), particularly in patients harboring ESR1 mutations.
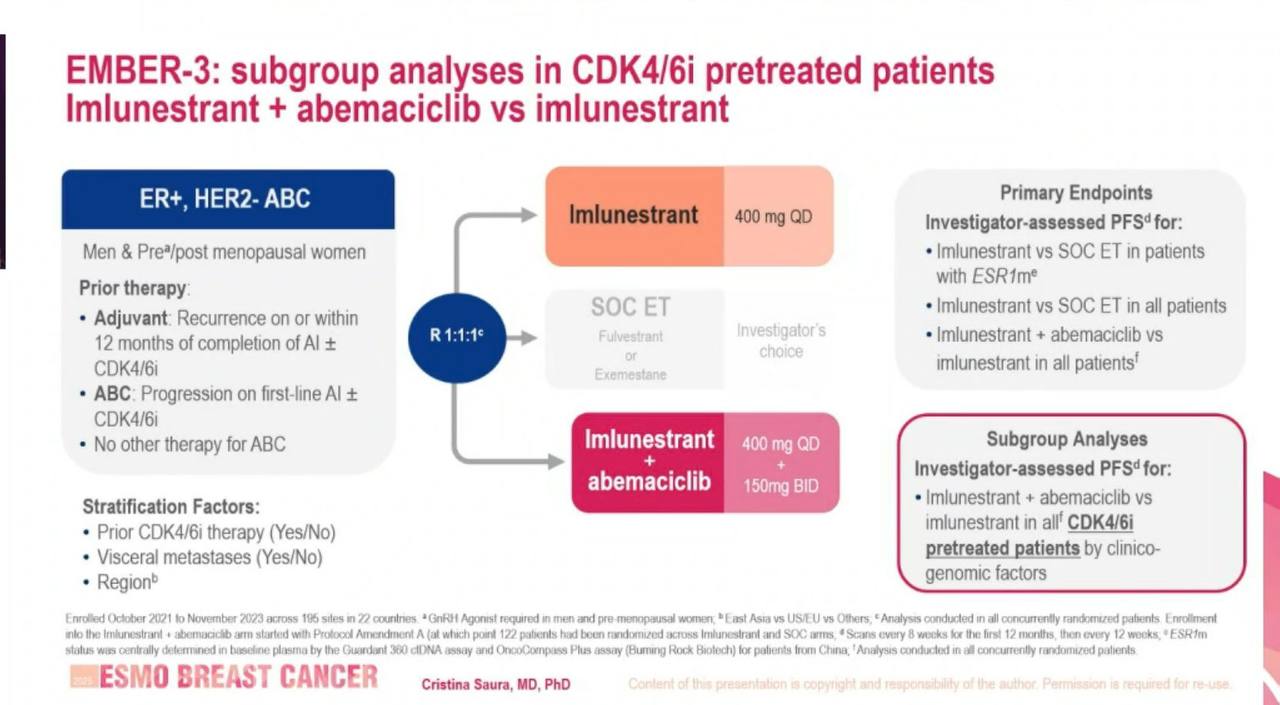
EMBER-3 Initial Trial Design and Enrollment
The phase III EMBER-3 trial (NCT04975308) enrolled 874 patients (As of Dec 2024) with locally confirmed ER+/HER2- advanced breast cancer that progressed during or after treatment with an aromatase inhibitor, with or without prior CDK4/6 inhibitor exposure. Key eligibility criteria included:
-
Measurable or bone-only disease (per RECIST v1.1)
-
No prior therapies for advanced/metastatic disease beyond endocrine therapy ± CDK4/6i
-
No symptomatic or untreated brain metastases or visceral crisis
Initially designed as a two-arm trial (imlunestrant vs. standard-of-care endocrine therapy), the protocol was amended to include a third arm of imlunestrant + abemaciclib. Patients were randomized 1:1:1 to:
-
Imlunestrant (400 mg orally daily)
-
Standard endocrine therapy (exemestane or fulvestrant)
-
Imlunestrant + abemaciclib (150 mg twice daily)
Subgroup Analysis in CDK4/6i-Pretreated Patients
In this updated analysis, 426 patients from the EMBER-3 trial were randomized to either imlu alone or imlu + abemaciclib. Notably, 65% (n=279) of these patients had received prior CDK4/6 inhibitor therapy—most commonly for ≥12 months—making this a clinically relevant population with endocrine-resistant disease.
Key baseline characteristics included
- 56% with visceral metastases
- 37% with ESR1 mutations
- 40% with PI3K-pathway alterations (PIK3CA, AKT1, or PTEN)
Key Findings Presented at ESMO Breast 2025
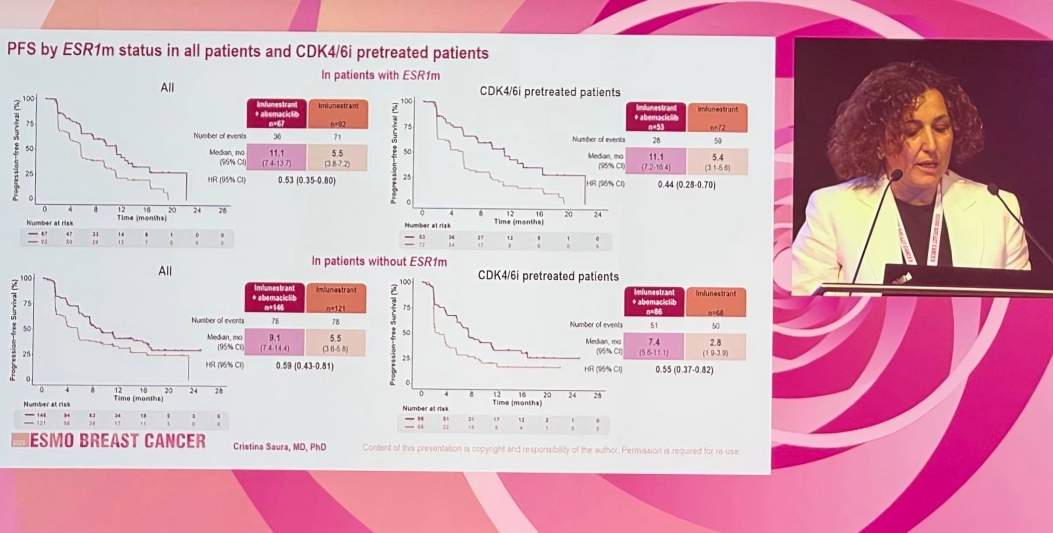
Across the entire CDK4/6i-pretreated population, the combination of imlu + abema demonstrated a 49% reduction in risk of progression compared to imlu monotherapy (HR 0.51; 95% CI, 0.38–0.68), with a median PFS of 9.1 vs 3.7 months, respectively.
This benefit was consistent across subgroups, including:
- Visceral metastases: HR 0.39
- Liver metastases: HR 0.38
- Bone-only disease: HR 0.57
- ESR1-mutant tumors: HR 0.44
- PI3K-pathway altered tumors: HR 0.52
- Concurrent ESR1 + PI3K alterations: HR 0.32
Even patients with longer prior CDK4/6i duration (≥12 months) saw meaningful benefit (HR 0.60), supporting the rationale for continued CDK4/6i use beyond progression when combined with a novel SERD. Notably, the analysis of patients previously treated with abemaciclib was limited due to small numbers.
You can read the previous full article published on December 11, 2024 in the New England Journal of Medicine

Key Takeaways from ESMO 2025
In patients with ER-positive, HER2-negative advanced breast cancer who experienced disease progression on prior CDK4/6 inhibitor therapy, the combination of imlunestrant and abemaciclib demonstrated a consistent and clinically meaningful progression-free survival benefit over imlunestrant alone. This benefit held true across a range of prespecified and clinically relevant subgroups, including those with visceral disease, liver metastases, and PI3K or ESR1 mutations.
The combination’s efficacy was independent of prior CDK4/6 inhibitor type or treatment duration, although analysis in patients previously treated with abemaciclib was limited by small numbers. Importantly, this is the first phase III trial to show benefit for an oral SERD plus CDK4/6 inhibitor after progression on CDK4/6i, offering a fully oral, targeted, and well-tolerated therapeutic option in a setting with few alternatives.
Overall survival data remain immature and are being followed. However, EMBER-3 establishes a new benchmark for post-CDK4/6i endocrine therapy in hormone receptor-positive breast cancer.
What They’re Saying: Reactions to EMBER-3 at ESMO Breast 2025
Jason A. Mouabbi, MD, Breast Medical Oncologist at MD Anderson Cancer Center, shared on X
Update from the #EMBER3 trial based on subgroup analysis
👀 at the curve in patients with co-mutated ESR1 and PIK3CA
Ahmed Alanazi, PharmD, BCPS, BCOP, Consultant Hem/Onc Pharmacist , Head of HEMONC Clinical Pharmacy shared on X
EMBER3 updates subgroups
Co mutation of PIK3CA/ESR1? and prior CDK4/6
Is Combo better than SERDs alone? Subgroups and low number of patients?
Combo showed more benefit! But >60% received palbociclib as prior CDK4/6i
Elisa Agostinetto, MD, Medical Oncologist, Jules Bordet Institute, Brussels, shared on X
At ESMOBreast25, CristinaSaura3 opens the proffered paper session presenting a subgroup analysis of EMBER-3 focusing on patients previously treated with CDK4-6i
Consistent benefit of imlunestrant + abema in this population
(Most pts had received prior palbo)
Philippe Aftimos, MD, Medical Oncologist, Clinical Trials Development Leader, Jules Bordet Institute, shared on X
At the proffered papers session 1, @CristinaSaura3 presents subgroup analyses from the EMBER-3:
Imlunestrant + Abemaciclib consistently improves median PFS vs Imlunestrant monotherapy in the subgroup of patients pretreated with CDK4/6i
You can Read The Full Abstract On ESMO Breast Cancer Congress 2025 Website