On May 14, 2025, the U.S. Food and Drug Administration (FDA) approved belzutifan (Welireg, Merck & Co., Inc.) for the treatment of adult and pediatric patients aged 12 years and older with locally advanced, unresectable, or metastatic pheochromocytoma or paraganglioma (PPGL). This marks a historic milestone as the first FDA-approved oral therapy for patients with these rare neuroendocrine tumors.
Key Evidence from LITESPARK-015 Trial
The FDA approval was based on results from LITESPARK-015 (NCT04924075), an open-label, multi-cohort, phase 2 trial. Cohort A1 specifically evaluated 72 patients with histologically confirmed PPGL that was unresectable or metastatic, and not suitable for curative therapy.
Patients had measurable disease per RECIST v1.1 as confirmed by blinded independent central review (BICR). Those with controlled hypertension were eligible, provided no recent changes in antihypertensive medications had occurred for at least two weeks. Patients with carcinomatous meningitis were excluded.
Efficacy Outcomes
- Objective Response Rate (ORR): 26% (95% CI: 17%–38%)
- Median Duration of Response (DOR): 20.4 months (95% CI: 8.3–NR)
- Among the 60 patients on antihypertensive therapy at baseline, 19 patients (32%) experienced a ≥50% reduction in at least one antihypertensive medication that was sustained for at least six months (95% CI: 20%–45%).
Safety Profile
Belzutifan was generally well tolerated, with side effects consistent with its known safety profile. The most common adverse reactions (≥25%), including laboratory abnormalities, were:
- Hematologic: Anemia, decreased lymphocytes, decreased leukocytes
- Hepatic: Increased ALT, AST, and alkaline phosphatase
- Musculoskeletal and constitutional: Fatigue, musculoskeletal pain
- Electrolyte disturbances: Increased calcium, increased potassium
- Others: Headache, dyspnea, dizziness, and nausea
Dosing and Administration
The recommended dosage for adults is 120 mg taken orally once daily. For pediatric patients aged 12 years and older, dosing is weight-based: those weighing 40 kg or more should receive 120 mg once daily, while those under 40 kg should receive 80 mg once daily. Treatment should be continued until disease progression or the development of unacceptable toxicity. This regimen is based on clinical data supporting consistent efficacy and tolerability across age and weight groups.
You can find detailed information about Belzutifan (Welireg) approvals on the official FDA website.
What is Belzutifan and How Does It Work?
Belzutifan (Welireg) works by targeting and inhibiting hypoxia-inducible factor 2-alpha (HIF-2α), a key protein that drives tumor growth and blood vessel formation in cancers linked to von Hippel-Lindau (VHL) gene mutations. By blocking HIF-2α, belzutifan disrupts cancer cell survival and proliferation, making it an effective treatment for VHL-associated tumors such as renal cell carcinoma, central nervous system hemangioblastomas, and pancreatic neuroendocrine tumors. This targeted mechanism helps control tumor growth with a manageable safety profile.
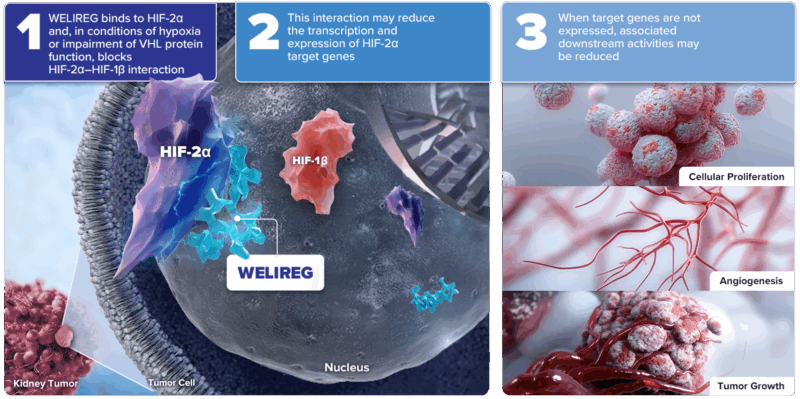
Photo from Welireg official website
Exploring the LITESPARK Trials: The Journey of Belzutifan
The FDA approved belzutifan in August 2021 for VHL-associated RCC, CNS hemangioblastomas, and pNETs based on LITESPARK-004. In December 2023, it was approved for advanced RCC after prior PD-1/PD-L1 and VEGF-TKI therapy, supported by LITESPARK-005 trial results.
The phase 2 LITESPARK-004 trial, published in the Journal of Clinical Oncology in 2022, evaluated belzutifan in patients with von Hippel–Lindau (VHL) disease–associated tumors. After over two years of follow-up, belzutifan achieved a 59% objective response rate (ORR) in renal cell carcinoma (RCC), 90% in pancreatic neuroendocrine tumors (pNETs), and 38% in CNS hemangioblastomas. Most responses were partial, with no new safety concerns. Only 3% of patients stopped treatment due to side effects. These results supported belzutifan’s approval as a systemic therapy for VHL-related tumors.
Published in the New England Journal of Medicine in 2024, the phase 3 LITESPARK-005 trial compared belzutifan to everolimus in 746 patients with previously treated advanced clear-cell renal-cell carcinoma. Both groups had a median progression-free survival (PFS) of 5.6 months. However, at 18 months, 24% of belzutifan-treated patients remained progression-free versus only 8.3% with everolimus (P=0.002). Belzutifan also achieved a higher objective response rate (21.9% vs. 3.5%, P<0.001). Median overall survival was 21.4 months with belzutifan vs. 18.1 months with everolimus, but this difference was not statistically significant. Belzutifan was well tolerated, with fewer treatment discontinuations and no new safety concerns.

In 2025, the Journal of Clinical Oncology published results from the phase 2 LITESPARK-015 study evaluating belzutifan monotherapy in Chinese patients with von Hippel-Lindau (VHL) disease–associated tumors. This single-arm trial enrolled 23 adults with localized VHL-related tumors, including renal cell carcinoma (RCC), central nervous system hemangioblastomas (CNS-HBs), pancreatic neuroendocrine tumors (pNET), and pheochromocytoma/paraganglioma (PPGL). All patients received oral belzutifan 120 mg once daily until disease progression or unacceptable toxicity.
The objective response rate (ORR) in patients with RCC (n=18) was 83%, with 15 partial responses. For CNS-HBs with solid components (n=7), ORR was 100%; for those with both solid and cystic components (n=10), ORR was 60%. In patients with pNET (n=12), ORR reached 67%. All four patients with PPGL had stable disease. The disease control rate (DCR) was 100% across all tumor types. Median time to response ranged from 2.8 to 5.6 months, and responses were ongoing at a median follow-up of 14.9 months. No patients experienced progression or death at the time of data cutoff.
Treatment-related adverse events were manageable. All patients experienced at least one adverse event, most commonly anemia, elevated liver enzymes, asthenia, upper respiratory infections, and increased GGT. Grade 3 adverse events occurred in 39% of patients, with no grade 4 or 5 events. Only one patient discontinued treatment due to a tumor hemorrhage.
These findings highlight the clinical benefit and safety of belzutifan for Chinese patients with localized VHL-associated tumors.
What is von Hippel-Lindau disease?
Von Hippel-Lindau disease is a rare genetic disorder caused by mutations in the VHL tumor suppressor gene. It increases the risk of developing multiple benign and malignant tumors across the body, including clear cell renal cell carcinoma (RCC), central nervous system (CNS) hemangioblastomas, retinal hemangioblastomas, pancreatic neuroendocrine tumors (pNET), and pheochromocytomas. These tumors often appear in young adulthood and can occur in multiple organs simultaneously. VHL is inherited in an autosomal dominant pattern, and early diagnosis is key to managing disease progression and preventing complications.
What is Pheochromocytoma?
PPGLs are rare neuroendocrine tumors that originate in adrenal or extra-adrenal tissue, often associated with excess catecholamine production leading to hypertension and related complications. Metastatic PPGLs have historically lacked effective systemic therapy options, especially oral treatments.
Pheochromocytoma is a rare neuroendocrine tumor that arises from chromaffin cells, typically located in the adrenal glands. When similar tumors occur outside the adrenal glands, they are called paragangliomas. Both can produce excess catecholamines (stress hormones), leading to symptoms like high blood pressure, headaches, rapid heartbeat, sweating, and anxiety. These tumors can be life-threatening if not diagnosed and treated early.
Pheochromocytomas are most often diagnosed between ages 30–50 and may be sporadic or linked to hereditary conditions such as MEN2, VHL, and NF1. Up to 30–40% are associated with genetic mutations, making genetic testing important for patients and their families.
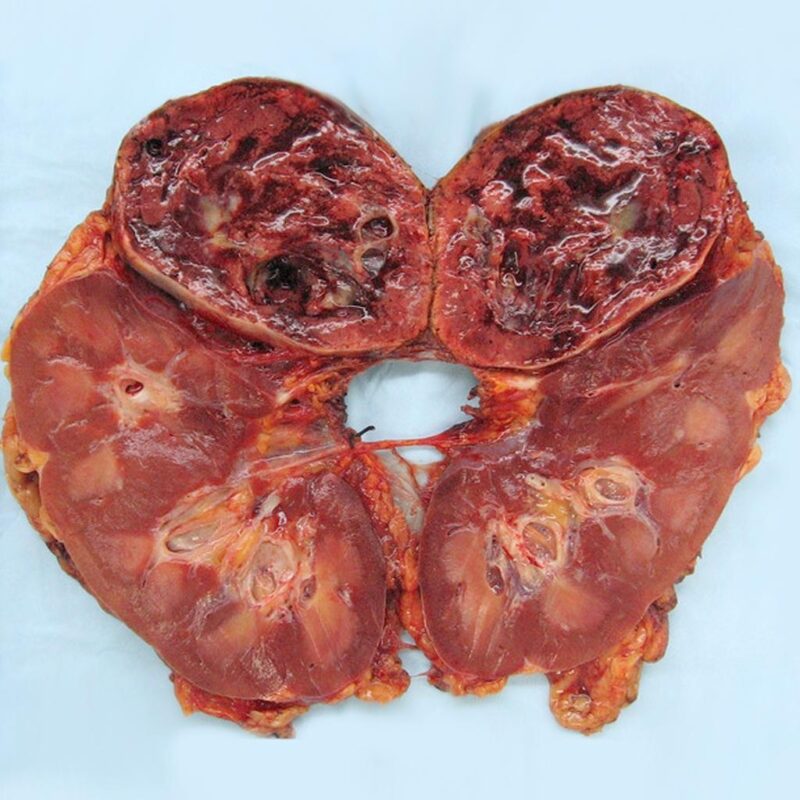
Gross pathology of pheochromocytoma. The image is taken from radiopaedia.org.
Diagnosis involves hormone level testing (plasma or urinary metanephrines) and imaging scans (CT, MRI, MIBG, or PET) to locate the tumor. Treatment usually involves surgical removal, often after pre-operative blood pressure control. Advanced or metastatic cases may need chemotherapy, radiation, or targeted therapy.
Long-term outlook is generally favorable with early treatment, but lifelong follow-up is essential to monitor for recurrence or new tumors. Patients may also face challenges such as hypertension, hormonal imbalances, and psychological stress. A multidisciplinary care approach improves outcomes and quality of life.
You can read about Pheochromocytoma in Adults on OncoDaily.


