
New Paper Alert: A pilot study on the neurocognitive effect of sorafenib on patients with desmoid tumors
Desmoid tumors (DTs) are rare, benign growths that can cause significant morbidity due to their tendency to grow locally and impact nearby structures. While surgical resection is often considered, recurrence rates are high, prompting the use of medical therapies such as sorafenib, a tyrosine kinase inhibitor. This study assessed the cognitive and emotional effects of prolonged sorafenib therapy in DT patients. By comparing patients on sorafenib, those not on treatment, and healthy controls, the research evaluated cognitive function, anxiety, and depression levels. Although no significant cognitive impairments were observed, the study found increased anxiety in patients on sorafenib, highlighting the need for mental health monitoring in this patient group.
Title: A pilot study on the neurocognitive effect of sorafenib on patients with desmoid tumors: the SORA-COG study
Authors: Kamboji Sharanya, Lulu Abdul khader, Fathima Shahama Oliyath Vazhayil, Ghazal Tansir, Vaishnavi Vishwas, Simran Kaur, Sameer Rastogi and Ratna Sharma
Published in ecancer in 13 Mar 2025
Background
Desmoid tumours (DTs) are rare benign growths, typically affecting individuals aged 25–40 years. These tumors can lead to swelling, pain, deformity, and, in severe cases, compromise the function of vital organs. While surgical resection remains a treatment option, the recurrence rate can be as high as 60%. As a result, observation and medical therapies are often recommended as first-line management. Sorafenib, a small molecule tyrosine kinase inhibitor (TKI), has emerged as a potential treatment for DT, though its impact on cognitive functioning, especially with prolonged use, remains underexplored.
Methods
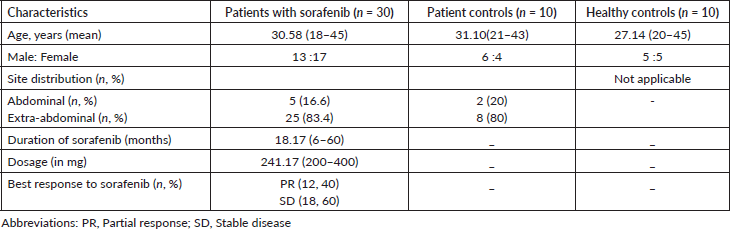
A cross-sectional study was conducted with 50 participants, including 30 patients with DT on sorafenib (Group 1), 10 patients with DT not on treatment (Group 2), and 10 healthy controls (Group 3). Participants were administered a variety of cognitive and emotional assessment tools, including the Appearance Anxiety Inventory (AAI), Depression Anxiety and Stress Scale (DASS-21), Hindi Mental Status Examination (HMSE), and a series of neuropsychological tests using the Cambridge Neuropsychological Test Automated Battery (CANTAB).
Study Design
The study was designed to assess cognitive function in patients with DT on prolonged sorafenib therapy. It included demographic and clinical data collection, along with detailed assessments of cognitive performance across multiple domains such as reaction time, memory, attention, and motor skills. Statistical analyses were conducted using non-parametric tests to assess the significance of differences between the groups.
Results
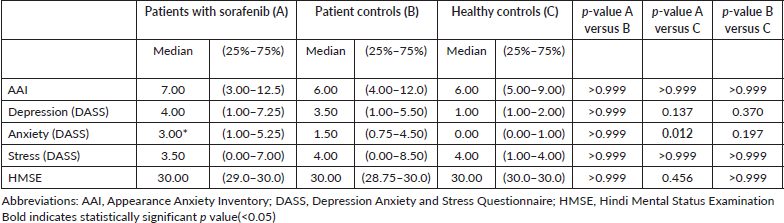
The study found no statistically significant differences in cognitive function scores across the three groups. Both subjective (AAI, DASS-21, HMSE) and objective (CANTAB) measures showed similar outcomes for patients on sorafenib, treatment-naïve patients, and healthy controls. However, there was a significant increase in anxiety levels in patients on sorafenib compared to healthy controls, suggesting that prolonged treatment may contribute to higher levels of anxiety, irrespective of treatment response.
Key Findings
Neurocognitive and emotional impacts of sorafenib treatment in desmoid tumors were elaborated in this study offers valuable insights into the cognitive and emotional impacts of sorafenib treatment in DT patients. Although no significant cognitive decline was observed, anxiety was notably elevated among those receiving sorafenib. These findings highlight the importance of monitoring mental health in patients on long-term TKI therapy.
- No significant cognitive impairment: Objective neuropsychological tests did not reveal significant cognitive declines in patients on sorafenib.
- Increased anxiety: Patients on sorafenib exhibited higher anxiety levels compared to both treatment-naïve patients and healthy controls.
- Correlation with treatment duration: The duration of sorafenib treatment was positively correlated with higher anxiety levels, suggesting that longer exposure may exacerbate anxiety.
Key Takeaway Messages
- Sorafenib treatment in DT patients does not seem to significantly affect cognitive functions, including memory, reaction time, and motor skills.
- Anxiety remains a notable concern, especially with prolonged sorafenib use, and warrants attention in clinical practice.
- Psychosocial support should be integrated into the care of DT patients undergoing long-term sorafenib therapy to address anxiety and improve overall well-being.
Summary By Sona Khachatryan, MD
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
