Fresh from the stage at ESTRO 2025, Prof. Leong Trevor has spotlighted the final results of the TOPGEAR trial, a major international phase 3 study evaluating the role of preoperative chemoradiotherapy (CRT) in resectable gastric and gastroesophageal junction (GEJ) adenocarcinoma. The findings, simultaneously published in the New England Journal of Medicine, contribute to a shifting paradigm in gastrointestinal oncology, as accumulating evidence continues to question the benefit of radiation in these settings.
 Trevor Leong at ESTRO 2025, Vienna
Trevor Leong at ESTRO 2025, Vienna
While the trial’s data were not favorable for radiation oncology, the study nonetheless provides critical insights into multimodal strategies and reaffirms the need for refined patient selection, robust quality assurance, and international collaboration.
Background of the TOPGEAR Trial
Gastric and GEJ cancers represent a global burden, with poor long-term outcomes despite advances in surgical and systemic therapies. In Europe and other Western regions, the FLOT regimen (fluorouracil, leucovorin, oxaliplatin, and docetaxel) is the current standard for perioperative chemotherapy. The potential benefit of adding radiotherapy has remained an open question.
The TOPGEAR trial (Trial of Preoperative Therapy for Gastric and Esophagogastric Junction Adenocarcinoma) was designed to directly assess whether adding preoperative chemoradiotherapy to standard perioperative chemotherapy could enhance outcomes, particularly overall survival (OS), pathological complete response (pCR), and local control.
Study Design and Methods
TOPGEAR was a randomized, open-label, multicenter, phase 3 trial conducted across 70 institutions in Australasia, Canada, and Europe from 2013 to 2020.
A total of 574 patients with resectable gastric or GEJ adenocarcinoma were randomized:
- Arm A (n=288): Perioperative chemotherapy alone (ECF or FLOT).
- Arm B (n=286): Preoperative chemoradiotherapy (45 Gy with concurrent fluorouracil) followed by perioperative chemotherapy.
The primary endpoint was overall survival. Secondary endpoints included pathological response, progression-free survival (PFS), adverse events, and surgical outcomes. The median follow-up was 67 months, providing robust long-term data.
Insights from ESTRO 2025 Presentation
At ESTRO 2025, Dr.Leong Trevor addressed the broader implications of the neutral survival results.
Results
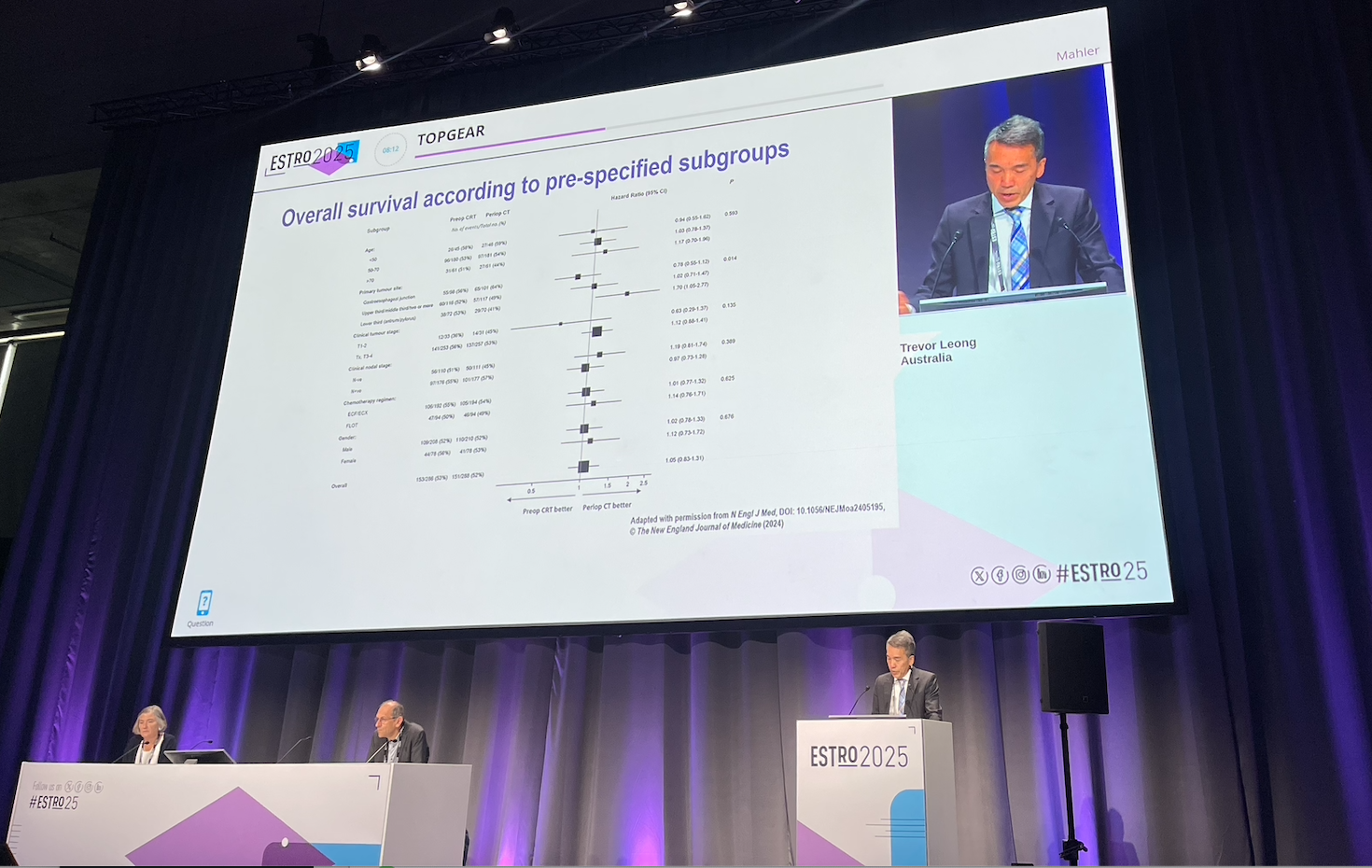
The TOPGEAR trial showed that both treatment approaches—perioperative chemotherapy alone and chemotherapy with preoperative chemoradiotherapy—were well tolerated and feasible, with high treatment completion rates and similar surgical outcomes. The addition of chemoradiotherapy led to improved tumor response and downstaging at surgery, suggesting better local disease control. However, this did not translate into a significant improvement in overall survival. Importantly, the addition of radiotherapy did not increase treatment-related toxicity or surgical risks, supporting its safety in this setting.
- Tumor location: 35% of tumors were at the GEJ
- Stage: 88% T3, 60% node-positive
- Compliance: 95% completed radiation, 91% completed chemotherapy
- Surgery performed: 84% in the chemoradiotherapy group, 89% in the chemotherapy group
- R0 resection rate: 92% in both groups
- Pathological complete response (pCR): 7.8% in chemoradiotherapy group vs. 0% in chemotherapy group
- Tumor downstaging and node-negative conversion were more frequent in the chemoradiotherapy group
- Median overall survival: 46 months in chemoradiotherapy vs. 49 months in chemotherapy — no significant difference
- Toxicity: Similar across groups with no major increase in surgical or GI/hematologic toxicities
Key Takeaways
- Although chemoradiotherapy improved tumor downstaging and pCR rates, it did not improve overall survival
- The trial confirms that chemoradiotherapy is safe and feasible but may not be necessary for all patients
- Findings were sometimes misrepresented in media and social platforms, which focused only on survival results and overlooked the benefits in local control
- The investigators stressed the importance of critically evaluating trial findings beyond headlines and called for personalized treatment approaches
- There is ongoing interest in whether chemoradiotherapy may play a greater role in selected cases, such as bulky or borderline resectable tumors
ESTRO 2025 Live Coverage by OncoDaily


