This article provides an in-depth exploration of medulloblastoma, a high-grade malignant brain tumor primarily affecting the cerebellum. It covers the tumor’s types, symptoms, diagnosis, and treatment strategies, with a particular focus on the latest 2025 advancements in research and care. The article delves into the molecular classification of medulloblastoma, highlighting the distinct genetic subgroups and their implications for prognosis and treatment. It also discusses the progress in personalized therapies, targeted treatments, and innovative approaches in radiation and chemotherapy, offering valuable insights into how these advances are improving outcomes for patients.
What Is Medulloblastoma?
Medulloblastoma is a type of embryonal tumor, which means it originates from cells that are immature or undifferentiated. These tumors are characterized by their rapid growth, tendency to spread throughout the central nervous system (CNS), and relatively poor prognosis if not treated promptly. They often arise in the posterior fossa, the part of the brain at the back of the skull, near the brainstem.
While most commonly affecting children, medulloblastoma can occur in adults as well. In pediatric cases, it most frequently presents between the ages of 3 and 8 years, with a slight male predominance.
Read More About Brain Cancer on Oncodaily
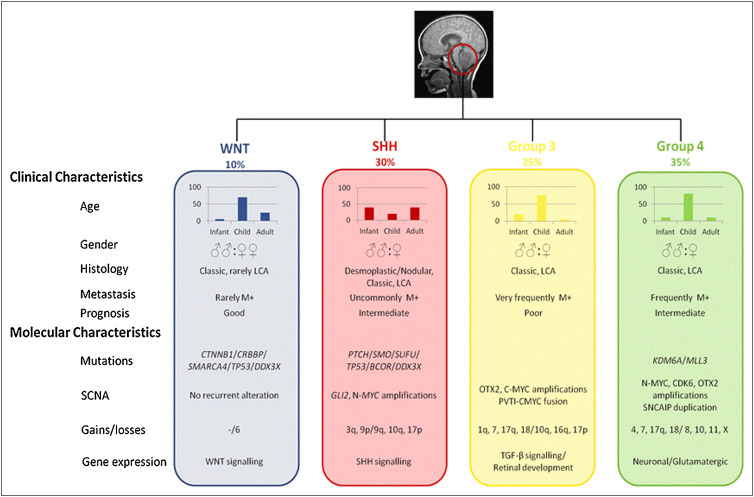
Types and Molecular Classification of Medulloblastoma
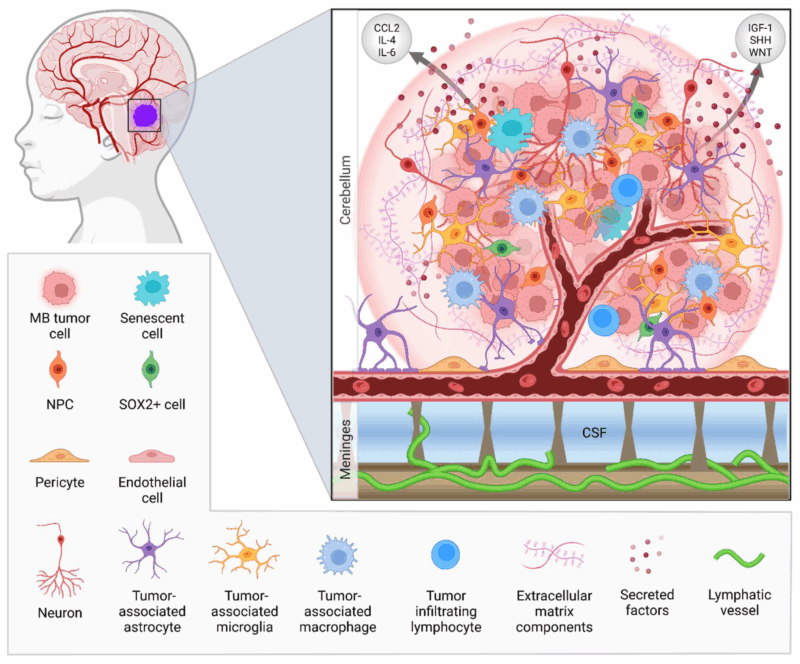
Medulloblastoma is classified into four molecular subgroups based on genetic and molecular characteristics. These classifications help predict prognosis and guide treatment decisions. The identification of these subgroups has significantly advanced the understanding of medulloblastoma and improved personalized therapy.
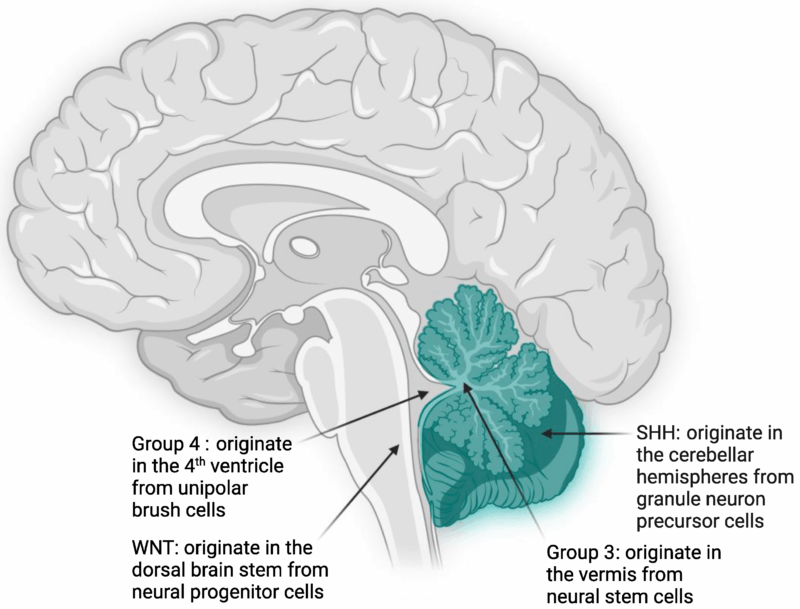
WNT-driven Medulloblastomas
WNT-driven medulloblastomas are characterized by mutations in the WNT signaling pathway, which plays a key role in cell growth and differentiation. These tumors typically show activating mutations in the CTNNB1 gene, which encodes beta-catenin, a critical component of the WNT signaling pathway. WNT-driven tumors have a favorable prognosis and are generally associated with a high rate of complete surgical resection and good response to radiation. They are more commonly diagnosed in adults and often show a lower rate of metastasis.
Sonic Hedgehog (SHH)-driven Medulloblastomas
SHH-driven medulloblastomas arise due to mutations in the Sonic Hedgehog (SHH) pathway, which regulates cell proliferation and differentiation during brain development. These tumors are often associated with PTCH1 mutations and other alterations in genes involved in the SHH pathway, such as SMO and SUFU. SHH-driven tumors have a more variable prognosis depending on the presence of additional mutations. They are typically found in children under the age of 5 and may involve cerebellar hemispheres or brainstem regions. These tumors are often more responsive to chemotherapy but may have a higher risk of recurrence if not completely resected.
Group 3 Medulloblastomas
Group 3 medulloblastomas are characterized by MYC amplification and other genetic abnormalities. These tumors are highly aggressive and often metastasize to the spinal cord and other areas of the central nervous system early in the disease course. They are more common in young children and are associated with a poor prognosis, even with aggressive treatment. Group 3 medulloblastomas tend to show rapid tumor growth, and patients often experience neurological deterioration despite chemotherapy and radiation. They are also more likely to develop recurrent disease after initial treatment.
Group 4 Medulloblastomas
Group 4 medulloblastomas are the most common subtype, but their molecular characteristics are less well understood than those of other groups. These tumors tend to have a complex array of genetic alterations, including changes in chromosome 17 and TP53 mutations. Group 4 medulloblastomas are typically less aggressive than Group 3 tumors and have a more favorable prognosis, but they still present a significant treatment challenge due to the risk of recurrence. Group 4 tumors are more often diagnosed in adolescents and young adults and are frequently associated with better survival outcomes when treated with a combination of surgery, radiation, and chemotherapy.
Importance of Molecular Classification
The molecular classification of medulloblastoma into these four subgroups has revolutionized how doctors approach diagnosis and treatment. For example, WNT-driven tumors have an excellent prognosis with minimal therapy, while Group 3 tumors require more aggressive treatments, and SHH-driven tumors often benefit from additional chemotherapy. This classification also helps identify patients who are at high risk of recurrence, enabling clinicians to tailor treatment protocols more effectively. Furthermore, the molecular subgroups guide clinical trials by providing a more nuanced understanding of how tumors behave and respond to different therapies, such as targeted therapies or immunotherapy.
In summary, the four molecular subgroups of medulloblastoma—WNT-driven, SHH-driven, Group 3, and Group 4—offer critical insights into the tumor’s behavior, prognosis, and response to treatment. Advances in molecular profiling have not only improved the accuracy of diagnosis but also paved the way for more personalized, targeted treatment strategies that enhance survival outcomes for patients with medulloblastoma.
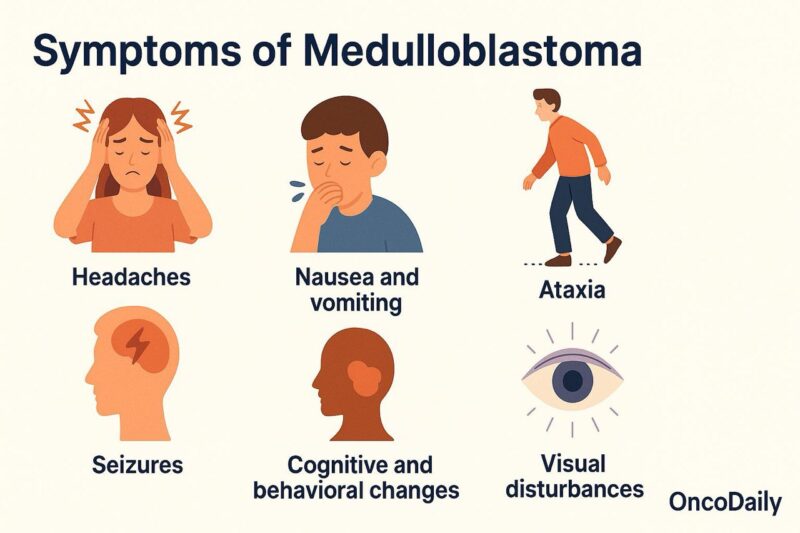
Symptoms of Medulloblastoma
Medulloblastoma symptoms primarily arise from the tumor’s location in the brain, particularly the posterior fossa, which can lead to increased intracranial pressure and disturbances in cerebellar function. The clinical presentation of medulloblastoma often develops gradually but can also appear suddenly depending on the tumor’s size and location.
Headaches are a common initial symptom, frequently worsening in the morning or with changes in position due to increased intracranial pressure. In many cases, headaches are accompanied by nausea and vomiting, both of which result from the build-up of pressure in the brain. These symptoms are often more pronounced when the tumor is large or growing rapidly.
Seizures are another prevalent symptom, occurring in approximately 50-60% of medulloblastoma patients. These can be focal or generalized, depending on the tumor’s spread within the brain. In children, especially, seizures may be the first sign prompting further investigation into possible brain involvement (Jallo & McComb, 2016).
As the tumor grows and infiltrates surrounding structures, it can affect motor coordination, leading to ataxia, which manifests as clumsiness, difficulty walking, and problems with balance. Since medulloblastomas often arise in the cerebellum, which is responsible for coordinating movement, ataxia is one of the hallmark symptoms of the condition (Gajjar et al., 2016).
Cognitive and behavioral changes are also common, particularly if the tumor spreads to the frontal lobe or other parts of the brain involved in cognitive function. These can include memory loss, changes in personality, and difficulty with concentration. Children with medulloblastoma may also experience developmental delays or regression in previously acquired skills. Behavioral changes, such as irritability or mood swings, may be more noticeable in younger patients (Khatua et al., 2016).
Visual disturbances, including blurred vision or visual field defects, can occur when the tumor compresses the optic pathways or interferes with the optic nerve. This symptom is more likely to manifest as the tumor grows and presses against surrounding structures (Wang et al., 2015).
Because medulloblastoma can affect multiple areas of the brain, the range of symptoms can vary widely, making early diagnosis and intervention crucial. The gradual onset of symptoms such as headaches, seizures, and motor impairments often leads to delays in diagnosis, as these signs can be attributed to other, less serious conditions. However, prompt recognition and imaging are essential to determine the extent of the tumor and begin appropriate treatment.
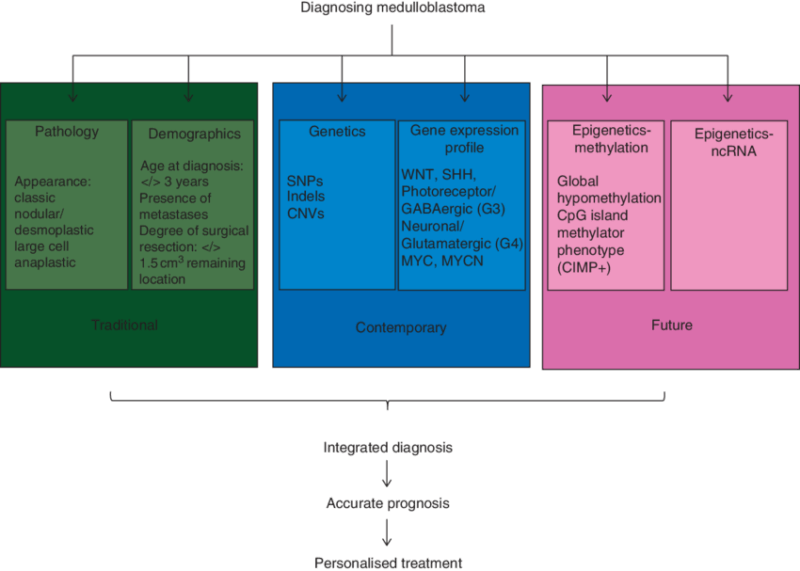
Diagnosis of Medulloblastoma
The diagnosis of medulloblastoma involves a comprehensive approach that combines clinical evaluation, neuroimaging, histopathological analysis, and molecular testing. A definitive diagnosis is critical, as it directly influences treatment strategies and the prognostic outlook for patients.
Clinical Evaluation
The diagnostic process typically begins with a detailed medical history and neurological examination. Patients often present with symptoms such as headaches, seizures, and balance problems, which raise suspicion for a brain tumor. The physician will assess the patient’s neurological function, including motor skills, coordination, and cognitive abilities, to understand the extent of the tumor’s effect on the brain.
Neuroimaging
Magnetic Resonance Imaging (MRI) is the gold standard for diagnosing medulloblastoma and assessing its size, location, and impact on surrounding brain structures. MRI scans typically reveal hyperintense lesions in T2-weighted and FLAIR sequences. Medulloblastomas often appear as well-circumscribed but infiltrative tumors, and contrast-enhanced T1-weighted images can show the tumor’s enhancement pattern, which varies based on the tumor’s grade and location.
- Cerebellar involvement: Medulloblastomas frequently affect the posterior fossa, causing obstructive hydrocephalus due to blockage of cerebrospinal fluid (CSF) flow, which may be seen on MRI as ventricular dilation.
- CT scans: In some cases, CT scans may be used to assess calcifications, which are commonly seen in medulloblastomas, though MRI is more sensitive for detecting tumor infiltration.
Biopsy and Histopathological Analysis
Once a brain tumor is identified through imaging, a biopsy or surgical resection is usually performed to obtain a tissue sample for histopathological analysis. Medulloblastomas are typically composed of small round blue cells, and characteristic features, such as the “fried egg” appearance of cells, are commonly identified. A detailed histological examination can help differentiate medulloblastoma from other pediatric brain tumors, such as ependymomas or gliomas.
Molecular Testing
Advances in molecular diagnostics have revolutionized the classification of medulloblastoma. Since 2016, the WHO has adopted a molecular classification system that divides medulloblastomas into four major subtypes based on genetic mutations and molecular features:
- WNT-driven Medulloblastomas: These tumors show WNT signaling pathway mutations, which are associated with a better prognosis.
- SHH-driven Medulloblastomas: These tumors are driven by mutations in the Sonic Hedgehog (SHH) pathway, and they can be further classified into groups based on specific genetic markers.
- Group 3 and Group 4 Medulloblastomas: These subtypes do not have a specific molecular driver like the others but are characterized by various genetic changes, such as MYC amplification (Group 3) or chromosomal instability (Group 4).
Molecular testing typically
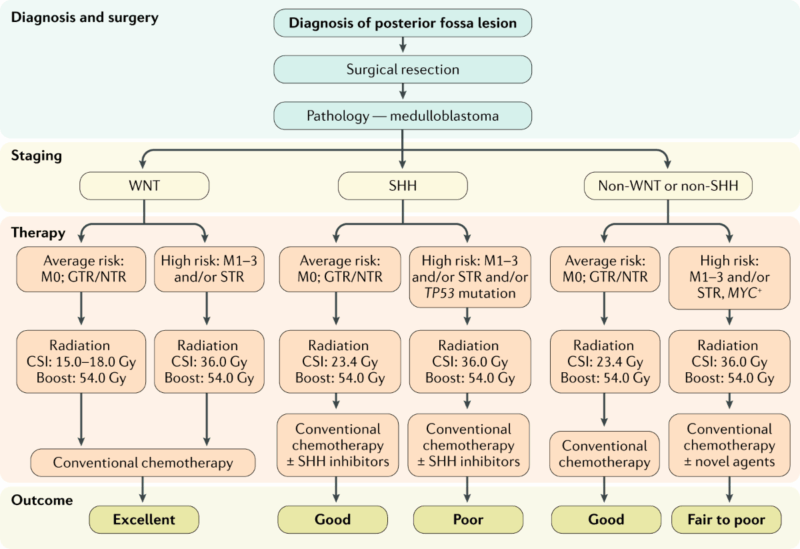
Treatment of Medulloblastoma
The treatment of medulloblastoma is multidisciplinary, typically involving surgery, radiation therapy, and chemotherapy. Treatment plans are guided by factors such as the tumor’s molecular subtype, the extent of resection, and the age of the patient. The main goals of treatment are to remove as much of the tumor as possible, prevent recurrence, and minimize long-term side effects, particularly in children who may have decades of survivorship ahead.
Surgical Resection
Surgical resection is the first line of treatment for medulloblastoma. The primary objective of surgery is to remove as much of the tumor as possible while minimizing damage to surrounding healthy brain tissue. Gross total resection, where the entire visible tumor is removed, is associated with the best prognosis and the lowest risk of recurrence. However, achieving complete resection can be difficult in tumors located in deep or critical brain regions, such as the posterior fossa, or if the tumor has spread extensively.
In cases where gross total resection is not possible, a partial resection may be performed, followed by radiation and chemotherapy to target any residual tumor tissue. Neurosurgeons will carefully plan the surgery to avoid damage to critical structures of the brain, such as the brainstem, cerebellum, and cranial nerves.
Radiation Therapy
Following surgery, radiation therapy is often used, particularly in high-risk patients or those with incomplete resection. Craniospinal irradiation (CSI), which involves delivering radiation to both the brain and spinal cord, is a standard treatment for medulloblastoma. This approach is used because medulloblastomas are known to spread throughout the CNS, and radiation to the entire craniospinal axis helps address any potential metastasis.
For pediatric patients, particularly those under 3 years old, who are more sensitive to the long-term effects of radiation, alternative treatment approaches may be used, such as reduced-dose radiation or the use of stereotactic radiosurgery for localized tumor control (Gajjar et al., 2016). Advances in targeted radiation techniques, like proton therapy, are being explored as ways to reduce the risk of neurocognitive side effects, particularly in younger patients.
Chemotherapy
Chemotherapy is a key component of the treatment plan for medulloblastoma, especially for patients with high-risk features, such as residual tumor after surgery or metastatic spread at the time of diagnosis. The most commonly used chemotherapy regimen is the vincristine, cisplatin, and cyclophosphamide (VCC) regimen, which has been shown to improve survival outcomes in pediatric patients when combined with radiation therapy. For children who are not candidates for full-dose radiation, chemotherapy can also serve as a substitute to help manage the tumor and prevent relapse.
Another commonly used chemotherapy regimen is methotrexate-based therapy, particularly for patients with high-risk or relapsed tumors. The combination of chemotherapy and radiation therapy significantly improves progression-free survival and overall survival, although it can lead to side effects such as hearing loss, growth impairment, and neurocognitive decline (Khatua et al., 2016).
Targeted Therapy and Molecular Approaches
Recent advances in molecular biology have led to the development of targeted therapies that focus on specific genetic and molecular features of medulloblastoma. For example, sonidegib, an inhibitor of the Sonic Hedgehog (SHH) pathway, has shown promise in treating medulloblastomas that are driven by SHH mutations. These tumors, while aggressive, can respond to targeted agents that inhibit the SHH signaling pathway, offering a more focused treatment with potentially fewer side effects compared to traditional chemotherapy.
Clinical trials are also investigating the role of IDH inhibitors, immunotherapy, and tumor-specific vaccines. For instance, the development of vaccines targeting mutant IDH or cancer testis antigens may hold promise for relapsed or refractory medulloblastomas. Immunotherapy, including immune checkpoint inhibitors, is under investigation for its potential to enhance the body’s immune response against medulloblastoma cells, particularly in high-risk patients.
Prognosis of Medulloblastoma
The prognosis of medulloblastoma depends on several key factors, including the tumor’s molecular subtype, histological grade, extent of surgical resection, the patient’s age, and their response to treatment. Advances in molecular profiling and a better understanding of the tumor’s genetic makeup have significantly improved the accuracy of prognostic predictions and personalized treatment strategies. Below is an overview of the main factors influencing the prognosis of medulloblastoma patients:
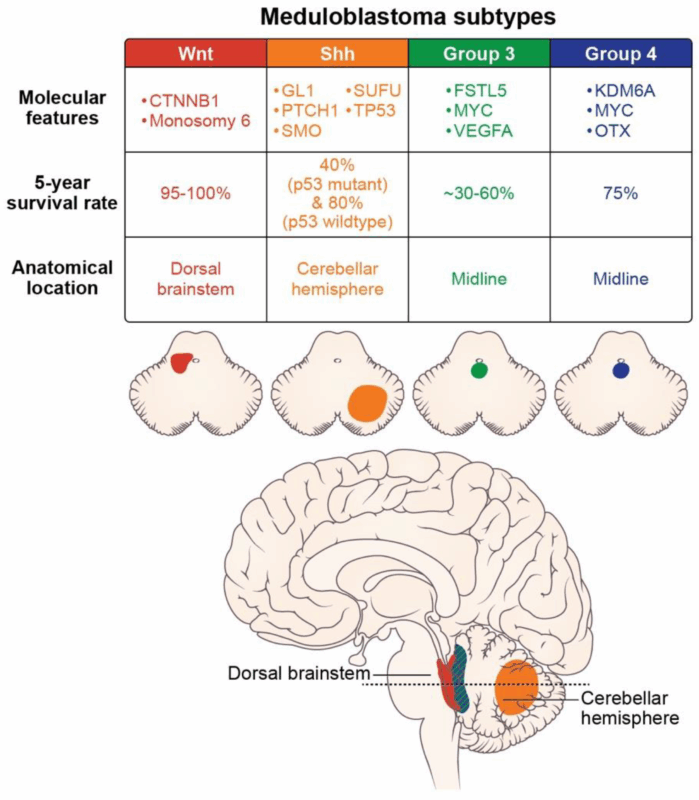
Tumor Subtype and Molecular Features
Molecular profiling has become an essential part of assessing the prognosis of medulloblastoma. The 2016 update of the World Health Organization (WHO) classification divided medulloblastomas into four major subtypes based on their genetic features: WNT-driven, SHH-driven, and Group 3 and Group 4 tumors. These molecular subtypes correlate closely with prognosis and guide treatment decisions.
WNT-driven medulloblastomas: These tumors tend to have a favorable prognosis due to their low rate of recurrence and high survival rates. They are typically associated with CNS tumors with very low metastatic potential, and the 5-year survival rate for this subtype is among the highest.
SHH-driven medulloblastomas: These tumors are characterized by alterations in the Sonic Hedgehog signaling pathway and can vary in their prognosis depending on additional genetic mutations and patient age. Infants and young children with SHH-driven tumors tend to have a better prognosis when treated early with a combination of surgery, radiation, and chemotherapy.
Group 3 medulloblastomas: These are often the most aggressive and carry the worst prognosis. They are frequently associated with MYC amplification, high metastatic potential, and resistance to treatment. However, early diagnosis and aggressive treatment can still lead to a 5-year survival rate improvement in certain cases.
Group 4 medulloblastomas: These tumors are the most common subtype and tend to have a relatively good prognosis compared to Group 3. However, their prognosis can vary based on factors like age, treatment response, and residual disease.
Age and Gender
The prognosis of medulloblastoma is also influenced by the patient’s age at diagnosis. Children under the age of 3 tend to have a poorer prognosis due to the difficulty of delivering effective radiation therapy at a young age. For children older than 3 years, the prognosis improves significantly, especially when the tumor is localized and surgically resected. Additionally, males tend to have a slightly higher risk of recurrence compared to females, though the reasons for this gender difference remain unclear (Ohgaki & Kleihues, 2005).
Extent of Surgical Resection
The extent to which the tumor is removed during surgery plays a crucial role in prognosis. Gross total resection—the removal of the entire visible tumor—has been consistently associated with a better prognosis and a lower likelihood of recurrence. Partial resection or biopsy, on the other hand, often requires additional treatment, such as radiation therapy and chemotherapy, and is linked with a higher risk of relapse. The degree of surgical resection should be carefully balanced with the need to preserve neurological function, especially when the tumor is located near critical brain structures (Gajjar et al., 2016).
Treatment Response
The response to treatment is a major determinant of prognosis. Patients who respond well to chemotherapy, radiation, or both, generally have a better long-term outlook. The most common chemotherapy regimens, such as vincristine, cisplatin, and cyclophosphamide (VCC), when combined with craniospinal radiation, have significantly improved survival outcomes for many patients. However, the prognosis for those who relapse or fail to respond to initial treatments is much less favorable, particularly in Group 3 medulloblastomas (Poussaint et al., 2011).
Long-Term Outcomes and Survivorship
Long-term survival is increasingly common in medulloblastoma patients, particularly with the advent of newer therapies and more precise radiation techniques. However, survivors often face long-term side effects from treatment. Neurocognitive impairments such as memory loss, difficulty concentrating, and learning disabilities are common in children who undergo craniospinal radiation. Additionally, endocrine dysfunction (e.g., growth hormone deficiency, hypothyroidism) is frequently observed among survivors. Monitoring for neurocognitive decline and endocrine abnormalities is an essential part of long-term survivorship care (Taphoorn et al., 2022).
Prognostic Markers
Several molecular and histological markers have been shown to influence prognosis in medulloblastoma. These include:
1p/19q codeletion: A specific genetic alteration often seen in Group 4 medulloblastomas, it is associated with a favorable prognosis.
MYC amplification: This is a poor prognostic factor, particularly in Group 3 tumors where high levels of MYC expression correlate with aggressive disease and a higher likelihood of metastasis.
TP53 mutations: These mutations, which affect tumor suppression, are more common in Group 3 and SHH-driven medulloblastomas, often indicating a worse prognosis.
MRI and CSF markers: MRI scans showing residual disease or evidence of metastasis significantly impact survival predictions. Likewise, markers of cerebrospinal fluid (CSF) involvement are important for predicting recurrence (Northcott et al., 2012).
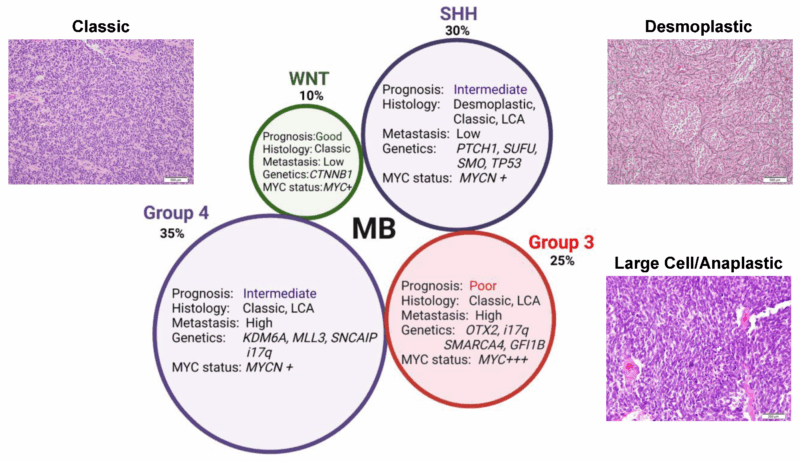
2025 Advances in Medulloblastoma Treatment
As of 2025, the treatment of medulloblastoma continues to evolve, with significant advancements being made in molecular profiling, immunotherapy, targeted therapies, and improved radiation techniques. Research is increasingly focused on personalized medicine, offering tailored treatments based on the tumor’s molecular characteristics and the individual patient’s needs. Below are key advancements that are shaping the future of medulloblastoma care:
Advances in Molecular Profiling and Classification
The incorporation of molecular genetics into the classification of medulloblastomas has transformed the way these tumors are diagnosed and treated. The 2021 WHO CNS tumor classification introduced the molecular subtypes of medulloblastoma, such as WNT-driven, SHH-driven, and Group 3 and Group 4 tumors. In 2025, more sophisticated genomic and transcriptomic profiling tools are being used to refine these subtypes and provide more personalized treatment options. As researchers learn more about the molecular drivers of tumor growth, therapies can be designed to target specific mutations, potentially improving survival rates and reducing side effects.
Additionally, liquid biopsy techniques, which detect circulating tumor DNA (ctDNA) and other biomarkers in blood or cerebrospinal fluid, are advancing as a non-invasive method for monitoring disease progression, detecting recurrence early, and assessing the effectiveness of therapy. This tool is gaining traction in clinical trials, offering the possibility of more frequent and less invasive monitoring for patients undergoing treatment (Piccioni et al., 2023).
Immunotherapy Breakthroughs
Immunotherapy, especially immune checkpoint inhibitors, has revolutionized the treatment of many cancers. However, medulloblastomas are often classified as immunologically “cold” tumors, meaning they exhibit lower immune cell infiltration and poor expression of immune checkpoint ligands like PD-L1. This has historically made them less responsive to conventional immunotherapies like nivolumab and pembrolizumab.
In 2025, research is focusing on strategies to “wake up” the immune system in medulloblastoma patients. For instance, combination therapies involving checkpoint inhibitors, targeted agents (such as IDH inhibitors), and radiation are being tested in clinical trials. These combinations aim to enhance the immune response against the tumor by reversing the immunosuppressive tumor microenvironment. Sonic Hedgehog (SHH) pathway inhibitors are also being tested in combination with immunotherapy to see if blocking this pathway enhances immune system recognition of the tumor (Platten et al., 2021).
Furthermore, tumor-specific vaccines, including IDH1-targeted vaccines and vaccines targeting cancer-testis antigens, are undergoing clinical trials. These vaccines are designed to elicit an immune response specifically against tumor cells, offering a promising new approach for relapsed or refractory medulloblastomas (Platten et al., 2021).
Read More About Immunotherapy for Brain Cancer on Oncodaily
Precision Radiation and Proton Therapy
While traditional craniospinal irradiation (CSI) has long been the standard treatment for medulloblastoma, it comes with significant long-term side effects, especially in children. In 2025, the focus has shifted toward precision radiation techniques, such as proton therapy and stereotactic radiosurgery (SRS), to minimize these toxicities while maintaining treatment efficacy.
Proton therapy, a form of particle beam therapy, is particularly beneficial for pediatric patients because it delivers targeted radiation that can spare healthy tissue and vital organs, especially in areas like the brainstem and cerebellum, which are critical for neurological function. Several trials are investigating proton therapy in combination with other therapies like chemotherapy or immunotherapy to improve overall survival rates while minimizing radiation-related damage (Yamada et al., 2018).
Stereotactic radiosurgery (SRS), a highly focused radiation treatment, is being evaluated in clinical trials as a means of targeting small areas of residual tumor or recurrence without subjecting the patient to the full-body effects of traditional CSI. This approach is especially useful for patients with localized recurrence or those who are not candidates for further surgery (Tsuchida et al., 2017).
Targeted Therapies for Subtypes
Targeted therapies are at the forefront of clinical research for medulloblastoma in 2025, particularly for tumors with distinct molecular signatures. For example, SHH-driven medulloblastomas, which make up a significant subset of cases, are being targeted with SHH pathway inhibitors like sonidegib and glasdegib. These agents aim to block the signaling pathways that mediate tumor cell proliferation, offering a targeted alternative to traditional chemotherapy.
MYC amplification in Group 3 medulloblastomas is another key area of research. These tumors are notoriously aggressive and are associated with poor prognosis. Clinical trials are testing MYC inhibitors and epigenetic modulators to prevent the expression of MYC and other oncogenes that drive tumor growth (Northcott et al., 2012). Additionally, IDH inhibitors are being explored for their potential to treat SHH-driven medulloblastomas by targeting the IDH mutations that produce 2-hydroxyglutarate (2-HG), a metabolite that contributes to tumor progression and immune evasion (Zhao et al., 2019).
Neurocognitive Preservation and Survivorship
As survival rates for medulloblastoma improve, there is increasing emphasis on the long-term effects of treatment. Neurocognitive decline, hearing loss, growth deficiencies, and endocrine dysfunction are common side effects of radiation and chemotherapy, particularly in pediatric patients. In 2025, clinical protocols are focusing on risk-adapted treatment approaches to reduce radiation doses in low-risk patients and incorporate cognitive preservation strategies.
Cognitive rehabilitation programs, growth hormone therapy, and endocrine monitoring are all part of the growing focus on survivorship care, ensuring that patients who survive medulloblastoma can lead a healthy, functional life as adults (Taphoorn et al., 2022).
Clinical Trials and New Drug Development
Ongoing clinical trials continue to push the boundaries of medulloblastoma treatment. Notable clinical trials include IDH inhibitors, SHH inhibitors, and checkpoint inhibitors in combination therapies. These trials aim to improve treatment outcomes, reduce toxicity, and prevent relapse, and are expected to set new standards for personalized treatment for medulloblastoma in the coming years.
Moreover, the incorporation of liquid biopsies in clinical trials is transforming how medulloblastoma recurrence is monitored. By tracking circulating tumor DNA (ctDNA), liquid biopsies offer a non-invasive means of detecting recurrence earlier than traditional imaging techniques like MRI (Piccioni et al., 2023).
You Can Watch More on OncoDaily Youtube TV
Written by Armen Gevorgyan, MD
FAQ
What is medulloblastoma?
Medulloblastoma is a type of brain tumor that originates in the cerebellum, a part of the brain responsible for coordination and balance. It is most common in children but can also occur in adults.
What are the symptoms of medulloblastoma?
Symptoms of medulloblastoma include headaches, nausea, vomiting, seizures, difficulty with balance and coordination (ataxia), cognitive and behavioral changes, and visual disturbances.
How is medulloblastoma diagnosed?
Diagnosis involves imaging tests such as MRI and CT scans, along with a biopsy for histological confirmation. Molecular profiling is also important for classifying the tumor into subtypes like WNT, SHH, Group 3, and Group 4.
What are the different types of medulloblastoma?
Medulloblastoma is classified into four molecular subgroups: WNT-driven, SHH-driven, Group 3, and Group 4. These classifications help predict prognosis and guide treatment decisions.
What is the treatment for medulloblastoma?
Treatment typically involves surgical resection of the tumor, followed by radiation therapy (including craniospinal irradiation) and chemotherapy. The treatment regimen depends on the tumor subtype, location, and the patient’s age.
How does molecular classification affect medulloblastoma treatment?
The molecular classification of medulloblastoma helps determine the most effective treatment. For instance, SHH-driven tumors may benefit from targeted therapies that inhibit the SHH pathway, while WNT-driven tumors have a more favorable prognosis with standard treatments.
What is the prognosis for medulloblastoma patients?
The prognosis varies based on the tumor subtype, the extent of resection, and the patient’s age. WNT-driven tumors generally have a good prognosis, while Group 3 tumors are more aggressive and associated with a poorer outlook.
What are the long-term effects of medulloblastoma treatment?
Survivors of medulloblastoma may experience long-term effects such as neurocognitive impairments, hearing loss, growth hormone deficiencies, and endocrine issues due to radiation and chemotherapy.
Are there any new treatments or research advancements for medulloblastoma?
Advances include targeted therapies for SHH-driven tumors, immunotherapies, and novel approaches to reduce radiation-related side effects, such as proton therapy. Ongoing research is also exploring the use of vaccines and immunotherapies.
Can medulloblastoma recur after treatment?
Yes, medulloblastoma can recur, especially in high-risk patients or those with incomplete resection. Regular follow-up with MRI scans is essential to monitor for recurrence, and clinical trials may offer new treatment options for relapsed cases.