The VIKTORIA-1 trial represents a pivotal advancement in addressing one of the most pressing challenges in hormone receptor–positive (HR+), HER2-negative (HER2–) advanced breast cancer (ABC): resistance to endocrine therapy (ET) and CDK4/6 inhibition (CDK4/6i). Such resistance remains a major therapeutic hurdle, as patients progressing after these standard treatments often experience limited benefit from subsequent options.
Central to this resistance is the activation of the PI3K/AKT/mTOR (PAM) signaling pathway, which drives tumor growth and survival. Gedatolisib, a potent dual PI3K/mTOR inhibitor, was developed to directly target this mechanism and potentially restore treatment sensitivity. In early-phase clinical studies, gedatolisib combined with palbociclib and fulvestrant demonstrated compelling efficacy, with a median progression-free survival (PFS) of 12.9 months and an objective response rate (ORR) of 56%.
These encouraging findings led to the initiation of the VIKTORIA-1 trial, a randomized, open-label, phase 3 study designed to evaluate gedatolisib-based regimens in patients with HR+/HER2– ABC whose disease had progressed after prior CDK4/6i plus aromatase inhibitor therapy. The study aimed to test whether targeting the PAM pathway could improve outcomes compared to endocrine therapy alone.
Methods
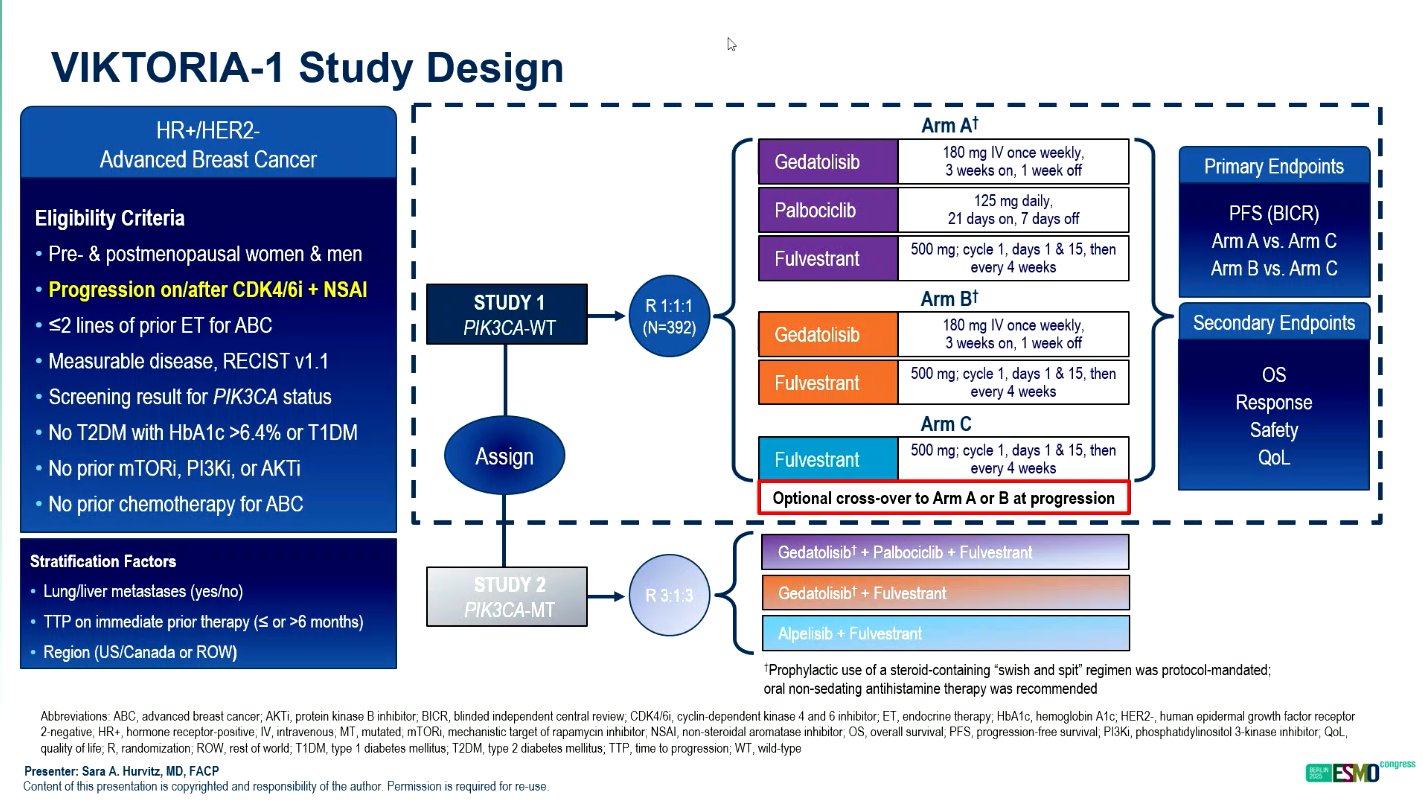
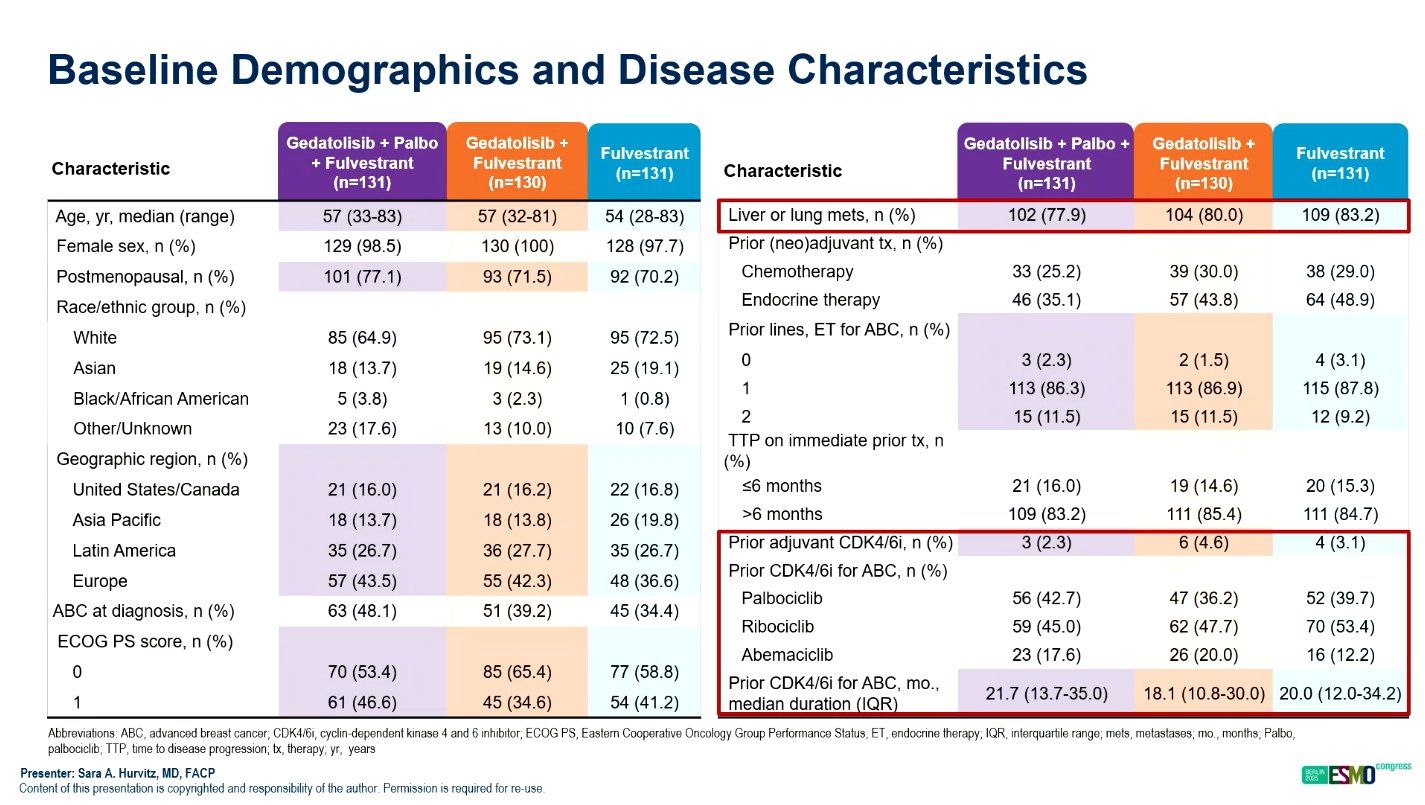
The VIKTORIA-1 trial (NCT055001886) is a randomized, open-label, phase 3 study that enrolled 392 patients with HR+/HER2–, PIK3CA wild-type advanced breast cancer following progression on CDK4/6 inhibitor therapy. Patients were randomized to one of three treatment arms in 28-day cycles: gedatolisib plus palbociclib plus fulvestrant (triplet), gedatolisib plus fulvestrant (doublet), or fulvestrant alone.
Doses were administered as follows: gedatolisib 180 mg intravenously weekly for three weeks per cycle, palbociclib 125 mg orally for 21 days, and fulvestrant 500 mg intramuscularly every two weeks for the first cycle and then every four weeks thereafter.
The co-primary endpoint was progression-free survival (PFS) assessed by blinded independent central review (BICR) for the triplet versus fulvestrant, followed by the doublet versus fulvestrant. Secondary endpoints included overall survival (OS), objective response rate (ORR), safety, and tolerability.
Results
At the time of data cutoff (May 30, 2025), the median study follow-up was 10.1 months. The VIKTORIA-1 trial met its primary endpoints, showing that both gedatolisib combinations significantly improved PFS compared with fulvestrant alone.
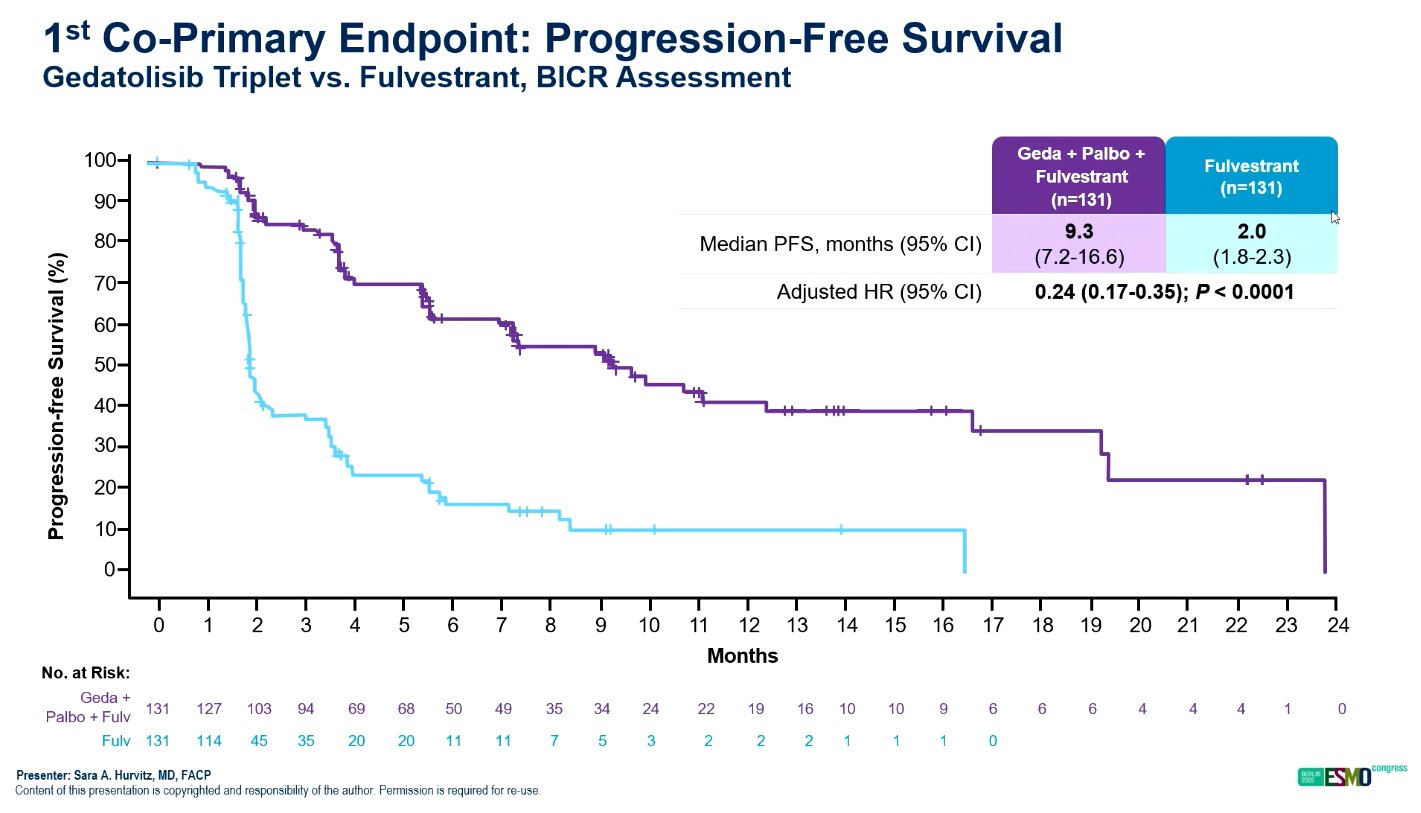
The median PFS was 9.3 months for the triplet, 7.4 months for the doublet, and 2.0 months for fulvestrant. These results translated to hazard ratios (HR) of 0.24 (95% CI, 0.17–0.35; P < 0.0001) for the triplet versus fulvestrant, and 0.33 (95% CI, 0.24–0.48; P < 0.0001) for the doublet versus fulvestrant. The findings represent a 76% and 67% reduction in the risk of disease progression, respectively.
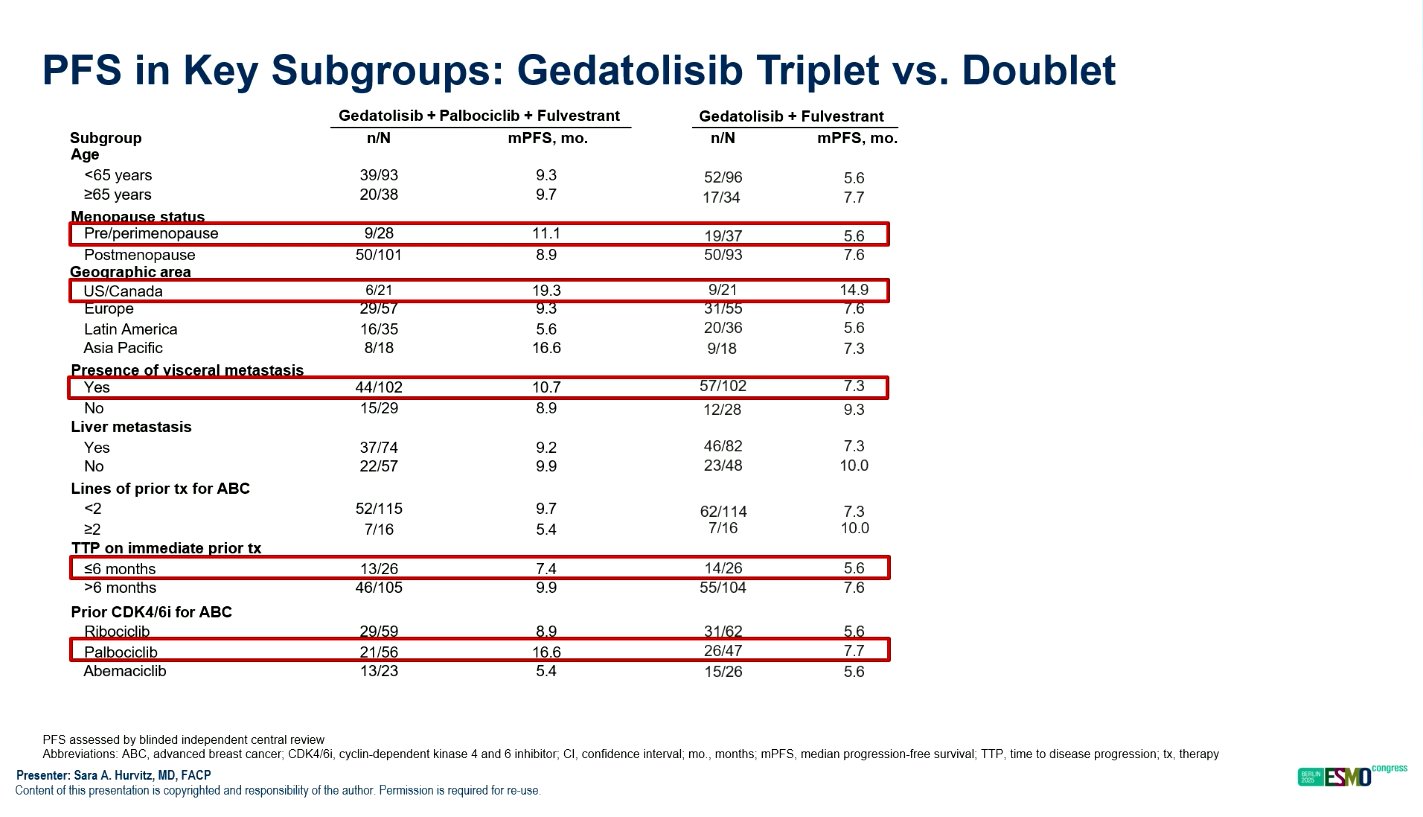
The benefit of gedatolisib-based therapy was consistent across all prespecified subgroups, including those defined by age, visceral disease, prior endocrine therapy response, and duration of CDK4/6 inhibitor use.
The objective response rate (ORR) was also higher in both gedatolisib arms, reaching 32% (including one complete response) in the triplet arm, 28.3% in the doublet arm, and only 1% in the fulvestrant group.
At the time of the interim overall survival (OS) analysis, a favorable trend was observed for the gedatolisib-containing regimens, with HR 0.69 (95% CI, 0.43–1.12) for the triplet versus fulvestrant and 0.74 (95% CI, 0.46–1.19) for the doublet versus fulvestrant. Although not yet statistically significant, these results suggest a potential survival advantage that warrants further follow-up.
Safety Profile
The safety profile of gedatolisib combinations was manageable and consistent with prior studies. Treatment discontinuation due to adverse events occurred in 2.3% of patients receiving the triplet and 3.1% receiving the doublet, indicating good overall tolerability.
The most common treatment-related adverse event was hyperglycemia, which occurred in 9.2% of patients on the triplet and 11.5% on the doublet, with grade 3 hyperglycemia reported in only 2.3% of patients in both arms. Other adverse events included fatigue, nausea, and diarrhea, which were mostly mild to moderate in severity.
No unexpected safety concerns were observed, and the incidence of serious adverse events was low. Importantly, the combination regimen did not introduce new toxicities beyond those already associated with individual agents.
Interpretation
The VIKTORIA-1 trial provides compelling evidence that gedatolisib-based regimens significantly improve progression-free survival compared with fulvestrant alone in patients with HR+/HER2–, PIK3CA wild-type advanced breast cancer after CDK4/6 inhibitor therapy.
The triplet regimen of gedatolisib, palbociclib, and fulvestrant achieved the most substantial benefit, more than quadrupling median PFS compared with fulvestrant and maintaining a favorable safety profile. These findings suggest that dual inhibition of PI3K and mTOR can effectively overcome endocrine resistance while maintaining tolerability, representing a promising therapeutic strategy in this challenging clinical setting.
Long-Term Follow-Up and Analytical Considerations
Although the median follow-up is currently just over ten months, the durability of responses and consistent PFS benefit across subgroups suggest that continued treatment may yield additional long-term advantages. The hierarchical testing design ensured statistical rigor and reliability of results, confirming the triplet’s superiority over endocrine therapy alone.
Future analyses will provide mature overall survival data and additional biomarker insights to identify patients who may derive the greatest benefit from PI3K/mTOR pathway inhibition. The low rate of discontinuation and manageable side effects support the integration of gedatolisib-based therapy into clinical practice once regulatory approvals are achieved.
Read Full Abstract on ESMO Congress 2025 Website
You Can Watch More on OncoDaily Youtube TV
Written by Armen Gevorgyan, MD


