The phase Ib/II TROPION-Lung02 trial (NCT04526691) explores this frontier by assessing the combination of Dato-DXd and pembrolizumab, with or without platinum-based chemotherapy, as first-line therapy for patients with metastatic or unresectable NSCLC. At ASCO 2025, investigators presented the largest dataset to date evaluating these regimens in untreated advanced NSCLC.
TROP2, a cell surface glycoprotein highly expressed in the majority of non-small cell lung cancers (NSCLC), has rapidly become a promising therapeutic target for innovative cancer treatments. The development of antibody-drug conjugates (ADCs) designed to exploit TROP2 expression, such as datopotamab deruxtecan (Dato-DXd), is ushering in a new era in NSCLC management. As the landscape of frontline treatment evolves, combining TROP2-directed therapies with immunotherapy offers a potentially more effective and durable strategy for patients with advanced disease.
Study Overview
TROPION-Lung02 is a multi-cohort, international clinical trial investigating the safety and efficacy of Dato-DXd in combination with pembrolizumab. The study enrolled 96 patients with previously untreated aNSCLC into two primary treatment arms: Dato-DXd plus pembrolizumab (doublet, n=42) and Dato-DXd plus pembrolizumab plus platinum chemotherapy (triplet, n=54). Both squamous and nonsquamous histologies were included, enhancing the generalizability of the findings.

Safety and Tolerability
Both regimens were generally well tolerated. The most frequent adverse events (AEs) were low-grade stomatitis (57% doublet, 33% triplet) and nausea (42% doublet, 48% triplet), consistent with known profiles of ADCs and platinum agents. Serious treatment-related AEs were observed in 12% of the doublet group and 22% of the triplet group, but no treatment-related deaths occurred.
Efficacy and Response
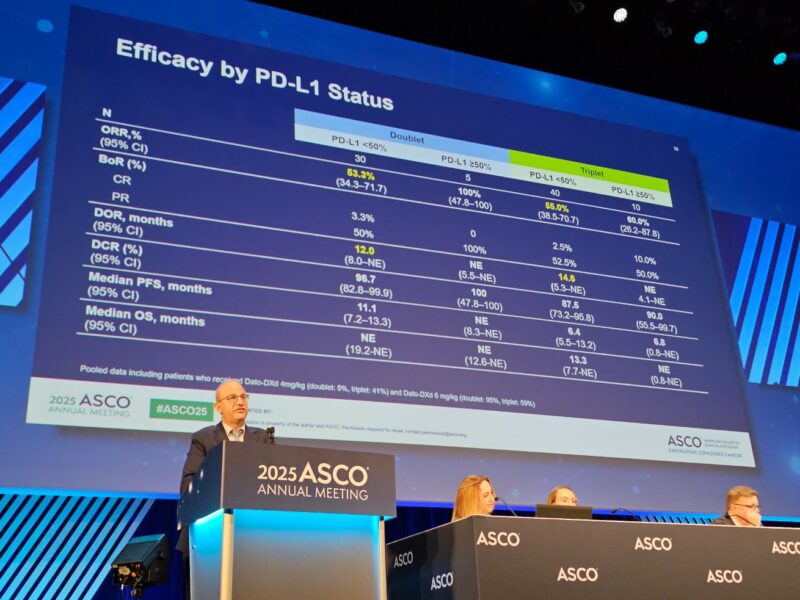
Both regimens produced robust confirmed objective response rates (ORR): 55% for the doublet and 56% for the triplet. Nonsquamous NSCLC patients saw ORRs of 52% (doublet) and 57% (triplet), while those with squamous histology had ORRs of 67% and 50%, respectively. Disease control rates (DCR) were also high across all groups—88–91%—demonstrating meaningful benefit for the majority of patients.
Durability of Response
Durable antitumor activity was seen, particularly with the doublet, which achieved a median duration of response (DOR) of 20.1 months. The triplet’s median DOR was 13.7 months. In nonsquamous disease, the doublet’s DOR reached nearly 25 months, suggesting this chemotherapy-free regimen could be especially valuable for select patients.
Progression-Free Survival
Median progression-free survival (PFS) for the doublet was 11.2 months, compared with 6.8 months for the triplet. For nonsquamous histology, PFS was 11.2 (doublet) and 10.8 (triplet) months, while in squamous NSCLC, it was 10.2 and 6.7 months, respectively.
Clinical Implications
TROPION-Lung02 underscores the potential for TROP2-directed therapies to transform frontline care in aNSCLC. The combination of Dato-DXd with pembrolizumab—both with and without platinum chemotherapy—was associated with high response rates, prolonged disease control, and manageable toxicity, regardless of tumor histology. For many patients, particularly those unable to tolerate chemotherapy, the doublet could offer a highly effective alternative.
These results set the stage for further research and may soon influence treatment guidelines as additional biomarker and long-term survival data emerge.
What They’re Saying: Reactions to TROPION-Lung02 at ASCO 2025
Hidehito HORINOUCHI, Medical Oncologist, NCC Japan, Lung Cancer Study Group shared on X
TROPION-Lung 02: Datopotamab deruxtecan plus pembrolizumab with or without platinum chemotherapy as first-line therapy for advanced NSCLC, ORR 54%, DOR 20.1m, PFS 11.2m
Conclusion
TROPION-Lung02 provides the largest clinical dataset to date evaluating the combination of a TROP2-directed antibody-drug conjugate, datopotamab deruxtecan (Dato-DXd), with pembrolizumab—with or without platinum-based chemotherapy—as first-line treatment in patients with advanced non-small cell lung cancer (NSCLC). Both doublet and triplet regimens elicited robust and durable antitumor activity, with confirmed objective response rates exceeding 50% and disease control rates close to 90% across nonsquamous and squamous histologies. Median progression-free survival (PFS) and duration of response were clinically meaningful, particularly in the doublet cohort, with extended follow-up. The safety profile was consistent with the known toxicities of each agent, and no new safety signals emerged.
These findings establish Dato-DXd plus pembrolizumab, with or without platinum chemotherapy, as a highly active and generally well-tolerated immunotherapy-based approach for previously untreated advanced NSCLC. The data support further investigation of this combination as a potential new standard of care in the frontline setting, especially for patients ineligible for standard platinum-based regimens or those seeking chemotherapy-free alternatives.
Key Takeaway Messages from TROPION-Lung02 Trial
Dato-DXd plus pembrolizumab, with or without platinum chemo, showed high response rates and durable antitumor activity in first-line advanced NSCLC.
Disease control rates were high and responses were sustained, especially with the doublet (Dato-DXd + pembrolizumab) regimen.
Safety profile was manageable and consistent with known effects of the drugs.
These results support further study of Dato-DXd combinations as a potential new frontline option in advanced NSCLC.
You Can Watch More on OncoDaily Youtube TV
Written by Armen Gevorgyan, MD


