January 2026 delivered a dense set of clinically and biologically meaningful advances in pancreatic cancer, spanning early-phase targeted therapies, randomized chemotherapy trials, translational biomarker studies, immuno-oncology mechanisms, perioperative risk assessment, surgical quality metrics, and medicinal chemistry innovation.
Across these updates, progress was observed along the full PDAC continuum—from molecular targeting of KRAS and DNA repair pathways, to immune resistance mechanisms, perioperative complication stratification, and refined prognostic subgroups in metastatic disease. Together, these studies reflect incremental but meaningful advances in treatment development, patient stratification, and outcome assessment in one of oncology’s most challenging malignancies.
Phase I INCB161734-101 Trial Updates
Early clinical data from the phase I INCB161734-101 trial (NCT06179160), presented at the 2026 ASCO Gastrointestinal Cancers Symposium (ASCO GI 2026), evaluate INCB161734, a selective oral KRAS G12D inhibitor, administered as monotherapy and in combination with standard-of-care chemotherapy in patients with advanced pancreatic ductal adenocarcinoma. In patients treated with INCB161734 1200 mg once daily monotherapy, treatment demonstrated a manageable safety profile and evidence of antitumor activity.
Reported results include:
- Disease control rate: 78% among evaluable patients
- Objective response rate: 37%, with several responses ongoing at data cutoff
- Durability: treatment durations extending beyond one year in a subset of patients
- Safety: treatment discontinuation due to treatment-related adverse events occurred in 3%, with predominantly low-grade gastrointestinal events that generally improved after the first cycle
- Combination therapy: INCB161734 was well tolerated with gemcitabine plus nab-paclitaxel or modified FOLFIRINOX, without compromising chemotherapy dose intensity
Overall, these data support continued clinical development of INCB161734 in pancreatic cancer.

Read about INCB161734 at ASCO GI 2026: Early Clinical Data in KRASG12D-Mutated Pancreatic Cancer on OncoDaily.
ISGPS Redefines Postpancreatectomy Mortality
Published in Annals of Surgery (January 23, 2026), this International Study Group for Pancreatic Surgery (ISGPS) consensus article introduces a standardized definition and classification of postpancreatectomy mortality to improve consistency, interpretability, and quality assessment in pancreatic surgery.
Postpancreatectomy mortality (PPM) is defined as death within 90 days of any pancreatic resection that is directly or indirectly attributable to a surgical complication, retrospectively linked through root-cause analysis. The ISGPS classification reflects distinct mechanisms:
- PPM 1: vascular injury / technical complexity–related mortality (15–30%)
- PPM 2: pancreatectomy-specific complication–related deaths, mainly postoperative pancreatic fistula (POPF)with secondary systemic deterioration (45–65%)
- PPM 3: cardiopulmonary or cerebrovascular deaths (10–25%)
Takeaway: By anchoring mortality to causality (not timing alone), this framework supports more meaningful quality assessment, benchmarking, and targeted strategies to reduce potentially preventable deaths after pancreatectomy.
Phase II TCOG T5217 Trial Updates
Results from the randomized phase II TCOG T5217 trial, published in the European Journal of Cancer on January 11, 2026, compare SLOG with modified FOLFIRINOX (mFOLFIRINOX) as first-line therapy in patients with locally advanced unresectable or metastatic pancreatic ductal adenocarcinoma.
No statistically significant differences were observed between the two regimens. Median progression-free survival was 7.5 months with SLOG versus 6.5 months with mFOLFIRINOX (HR 1.03; p = 0.88), and median overall survival was 12.9 versus 12.1 months, respectively (HR 1.04; p = 0.83).
Key findings included:
- Objective response rate: 38.5% with SLOG and 26.6% with mFOLFIRINOX, without a significant difference
- Safety: grade 3–4 neutropenia was more frequent with mFOLFIRINOX (53.1% vs 15.4%), while SLOG was associated with higher rates of selected non-hematologic toxicities
- Exploratory biomarkers: homologous recombination deficiency (HRD) was identified in 12.9% of patients and was associated with improved PFS and OS across both treatment arms
Overall, TCOG T5217 shows similar efficacy between SLOG and mFOLFIRINOX in advanced PDAC, with distinct toxicity profiles and supportive evidence for the prognostic relevance of HRD.
Read more about TCOG T5217 Phase 2 trial Updates on OncoDaily.
TIMP-1 Drives NK Cell Suppression in PDAC
Published in Cell Reports Medicine (January 20, 2026), this open-access study reports a comprehensive multimodal analysis identifying a cancer-immunoinstructive secretory signature (CISS) as a key driver of immunosuppression across human cancers, with particular relevance in pancreatic ductal adenocarcinoma (PDAC).
Using integrated single-nucleus and bulk transcriptomics, proteomics, functional assays, and clinical datasets, the authors show that CISS arises early during pancreatic inflammation and pre-malignancy, intensifies with tumor progression, and peaks in aggressive basal-like PDAC. The signature is quantitatively dominated by TIMP-1, which is causal for PDAC-cell–mediated suppression of natural killer (NK) cell function.
Key findings include:
- High CISS/TIMP-1 expression correlates with poor survival across multiple cancer types
- TIMP-1 suppresses NK cell cytotoxicity via CD74 signaling, impairing IL-2 responses and mTOR activity
- TIMP-1/CISS identify a high-risk basal-like PDAC subgroup
- Combined trametinib + nintedanib therapy suppresses TIMP-1/CISS and restores NK cell activity in preclinical PDAC models
Takeaway: This study defines a TIMP-1–dominated CISS as an early and actionable mechanism of tumor-driven immunosuppression, providing a strong biological and translational rationale for targeting the TIMP-1/CISS axis in PDAC and other aggressive cancers.
NEOLAP Trial Updates
A translational biomarker analysis from the phase II NEOLAP trial (AIO-PAK-0113), published in ESMO Gastrointestinal Oncology in 2026, evaluated growth differentiation factor-15 (GDF-15) as a prognostic and predictive biomarker in patients with locally advanced, non-metastatic pancreatic ductal adenocarcinoma treated with multiagent induction chemotherapy.
Low baseline circulating GDF-15 (≤0.8 ng/ml) was associated with longer overall survival (21.9 vs 12.7 months) and higher secondary R0 resection rates (36.5% vs 13.9%). Circulating GDF-15 levels increased during induction chemotherapy—more pronounced with platinum-based regimens—but these changes were not associated with treatment response or resection status.
Overall, NEOLAP identifies baseline GDF-15 as a clinically relevant marker for risk stratification and surgical outcome association in localized PDAC, supporting further evaluation in future trials.
Read more about NEOLAP Trial Updates on OncoDaily.
RAD51–BRCA2 Inhibition Sensitizes PDAC to Olaparib
Published in ACS Medicinal Chemistry Letters (January 26, 2026), this medicinal chemistry study reports the development of a new small-molecule series targeting the RAD51–BRCA2 protein–protein interaction to pharmacologically induce homologous recombination deficiency and enhance synthetic lethality with PARP inhibition in pancreatic cancer.
From this series, compound 19 emerged as a lead inhibitor that disrupts RAD51–BRCA2 binding, suppresses homologous recombination, and synergizes with olaparib in BRCA2-functional pancreatic cancer models, including both 2D cultures and 3D spheroids.
Key findings include:
- Inhibition of RAD51–BRCA2 interaction and reduced RAD51 foci formation
- Enhanced DNA damage and apoptosis with the 19/olaparib combination
- No significant cytotoxicity in normal pancreatic epithelial cells under the tested conditions
Takeaway: This work provides proof of concept that pharmacologic disruption of the RAD51–BRCA2 interaction can functionally induce homologous recombination deficiency (BRCAness) and sensitize pancreatic cancer cells to PARP inhibitors, positioning compound 19 as a valuable tool compound for further optimization and combination strategies in PDAC.
TWINPEAK study
Early clinical data from the ongoing TWINPEAK study (NCT05482893), reported as a Trial Update on OncoDaily in January 2026, evaluate spevatamig, an anti-CLDN18.2/CD47 bispecific antibody, in combination with gemcitabine and nab-paclitaxel as first-line therapy in CLDN18.2-positive metastatic pancreatic ductal adenocarcinoma.
In the 2 mg/kg weekly spevatamig + GnP cohort (n=15), the disease control rate was 93% and the objective response rate was 40%, with median PFS of 7.3 months and median OS of 13.2 months (maturing). Treatment was well tolerated, with no cytokine release syndrome, no grade ≥3 anemia, neutropenia, or thrombocytopenia, and no grade ≥3 nausea or vomiting at this dose level.
Responses were observed across CLDN18.2 expression levels ≥10%, with no apparent correlation between expression score and response. Overall, these data provide early clinical proof-of-concept supporting continued evaluation of spevatamig in combination with standard chemotherapy in first-line metastatic PDAC.
Read more about TWINPEAK study on OncoDaily.
Thromboembolic Events During Perioperative Therapy in PREOPANC-2
Published in the Journal of Clinical Oncology on January 29, 2026, this analysis from the phase III PREOPANC-2 trial evaluates the incidence and prognostic impact of venous thromboembolism (VTE) in patients with resectable and borderline resectable pancreatic ductal adenocarcinoma undergoing perioperative treatment.
Among 325 patients, VTE occurred in 28 patients (9%) within 12 months of random assignment, including 9 events preoperatively (3%) and 19 events postoperatively (8%). Most events were symptomatic (54%). Postoperative VTE was more frequent in the chemoradiotherapy arm than in the FOLFIRINOX arm (12% vs 3%, P = .02), although the 12-month cumulative incidence did not significantly differ between arms (11% vs 6%, P = .06). Two patients died from pulmonary embolism, both in the chemoradiotherapy arm. Importantly, VTE was independently associated with worse overall survival (adjusted time-varying HR 2.13, P = .002).
Takeaway: In PREOPANC-2, thromboembolic events affected nearly 1 in 10 patients undergoing perioperative treatment for (borderline) resectable PDAC and were associated with significantly reduced survival, underscoring the need for standardized VTE reporting and further evaluation of thromboprophylaxis during neoadjuvant therapy.
Lung-only metastatic pancreatic cancer
A retrospective single–academic-center study published in the European Journal of Cancer in 2026 analyzed 1012 patients with metastatic pancreatic ductal adenocarcinoma. Lung-only metastases were present in 109 patients (11%), defining a distinct subgroup. Compared with other metastatic patterns, lung-only patients were more often female, older at metastatic diagnosis, and less likely to present with synchronous metastases.
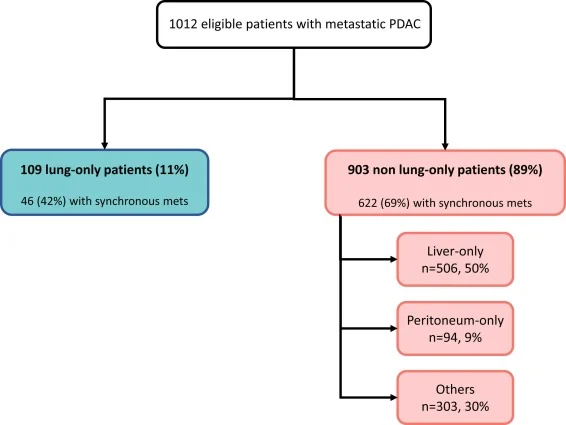
Median overall survival was significantly longer in lung-only PDAC (28.7 months) compared with liver-only (13.5 months), peritoneal-only (11.5 months), and other or multiple metastatic sites (11.3 months; p < 0.0001). Lung-only status remained an independent favorable prognostic factor in multivariable analysis (HR 0.40). Liquid biopsy showed lower detection of KRAS and TP53 mutations in lung-only patients, while no differences were observed when molecular profiling was performed on tumor tissue, consistent with reduced ctDNA shedding rather than distinct tumor genomics. Selected lung-only patients underwent curative-intent local treatments, which were associated with prolonged survival, although selection bias cannot be excluded.
Heavy Alcohol Consumption Increases Risk of Young-Onset Pancreatic Cancer
Published in the Journal of Clinical Oncology (January 23, 2026), this nationwide Korean cohort study evaluated the association between alcohol consumption and the risk of young-onset pancreatic cancer among adults aged 20–39 years.
In a cohort of 6.26 million individuals followed for up to 12 years, heavy alcohol consumption (≥30 g/day in men, ≥16 g/day in women) was associated with a significantly increased risk of young-onset pancreatic cancer (adjusted HR 1.19), whereas light-to-moderate intake was not. Risk increased in a threshold-dependent manner, particularly with alcohol consumption on ≥3 days per week.
The association was consistent across most subgroups after adjustment for smoking, pancreatitis, obesity, and metabolic comorbidities. These findings identify heavy alcohol consumption as a modifiable risk factor for young-onset pancreatic cancer and support targeted prevention strategies in young adults.