Pancreatic ductal adenocarcinoma (PDAC) remains a highly lethal malignancy, with most patients presenting with unresectable or metastatic disease and limited long-term survival despite combination chemotherapy. Intensive first-line regimens such as FOLFIRINOX have improved outcomes but are associated with substantial toxicity, prompting the development of modified schedules and alternative triplet combinations, particularly in Asian populations.
S-1–based regimens have demonstrated clinical activity and favorable tolerability in East Asia, leading to the development of the SLOG combination, which integrates oral S-1 and leucovorin with oxaliplatin and gemcitabine. The Taiwan Cooperative Oncology Group designed the TCOG T5217 trial to directly compare SLOG with modified FOLFIRINOX (mFOLFIRINOX) as first-line therapy for advanced PDAC, while also incorporating exploratory genomic analyses to assess the clinical relevance of homologous recombination deficiency. The results of the randomized phase II TCOG T5217 trial were published in the European Journal of Cancer on January 11, 2026.
Title: SLOG versus Modified FOLFIRINOX as First-Line Treatment for Advanced Pancreatic Cancer: A Randomized Phase II Trial (TCOG T5217)
Authors: Nai-Jung Chiang ∙ Yung-Yeh Su ∙ I-Wei Ho ∙ Li-Yuan Bai ∙ Chung-Pin Li ∙ Jen-Shi Chen ∙ Chin-Fu Hsiao ∙ Hsiao-Hui Tsou ∙ Chiun Hsu ∙ Tai-Jan Chiu ∙ Yao-Yu Hsieh ∙ Kun-Ming Rau ∙ Ching-Liang Ho ∙ Yan-Shen Shan ∙ Li-Tzong Chen
Methods
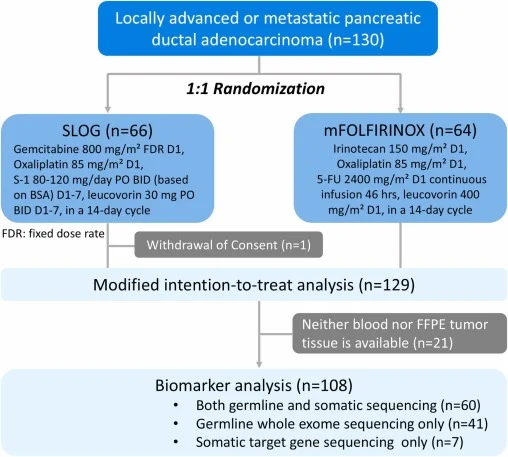
TCOG T5217 was an open-label, randomized, phase II multicenter study enrolling patients with histologically confirmed locally advanced unresectable or metastatic PDAC. Patients were randomized 1:1 to receive either SLOG or mFOLFIRINOX. Randomization was stratified by ECOG performance status, disease stage, and tumor location.
The primary endpoint was progression-free survival (PFS). Secondary endpoints included overall survival (OS), objective response rate (ORR), safety, and exploratory biomarker analyses. Tumor assessments were performed every eight weeks using RECIST 1.1, and adverse events were graded according to NCI-CTC version 4.0. Germline and somatic genomic profiling was prospectively incorporated to evaluate HRD status.
Results
Between March 2018 and November 2019, a total of 130 patients were enrolled across participating centers, with 129 included in the modified intention-to-treat population. Baseline characteristics were generally well balanced between the SLOG and modified FOLFIRINOX arms, although differences in primary tumor location were noted.
After a median follow-up of 37.7 months, efficacy outcomes were comparable between the two treatment strategies. No statistically significant differences were observed for the primary or key secondary endpoints.
Key efficacy findings included:
- Progression-free survival (PFS): median 7.5 months with SLOG versus 6.5 months with mFOLFIRINOX
- Overall survival (OS): median 12.9 months versus 12.1 months, respectively
- Objective response rate (ORR): 38.5% with SLOG and 26.6% with mFOLFIRINOX, without a significant difference
There were no statistically significant differences in PFS (HR 1.03; p = 0.88) or OS (HR 1.04; p = 0.83). Tumor response patterns were consistent across disease stages, with no meaningful interaction between treatment arm and locally advanced versus metastatic disease.
Exploratory biomarker analyses provided clinically relevant insights. Among 108 patients with available genomic data, homologous recombination deficiency (HRD)–related alterations were identified in 14 patients (12.9%), including both germline and somatic events.
Patients with HRD mutations demonstrated significantly improved outcomes compared with HRD–wild-type patients:
- Median PFS: 11.9 vs. 7.0 months
- Median OS: 17.7 vs. 11.7 months
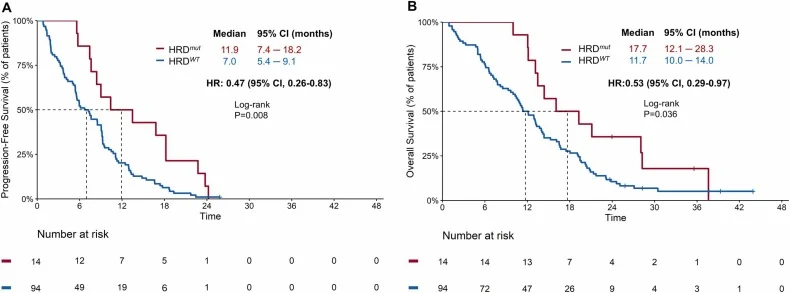
This survival advantage was observed consistently across both treatment arms and appeared numerically greater, although the study was not powered to formally test differences between germline and somatic HRD subgroups.
Treatment exposure and dose intensity were comparable to prior Asian triplet-chemotherapy studies. Dose reductions were common in both arms, reflecting real-world tolerability of intensive regimens.
Safety
Safety profiles differed substantially between treatments. Grade 3–4 neutropenia was significantly more frequent with mFOLFIRINOX (53.1% vs 15.4%; p < 0.001). SLOG was associated with higher rates of selected non-hematologic toxicities, including significantly increased skin hyperpigmentation, while grade 3–4 oral mucositis was numerically higher but not statistically significant. As part of post-study management, a subset of patients (14 patients, 10.9%), more frequently in the SLOG arm (10 vs 4), achieved sufficient disease control to undergo conversion surgery, and the majority received subsequent systemic therapy following study treatment, reflecting contemporary treatment sequencing.
Conclusion
The randomized phase II TCOG T5217 trial demonstrates that SLOG does not confer superior efficacy over modified FOLFIRINOX as first-line therapy for advanced pancreatic ductal adenocarcinoma. Nonetheless, the study highlights important differences in toxicity profiles and provides robust exploratory evidence supporting the prognostic relevance of homologous recombination deficiency in PDAC.
While mFOLFIRINOX remains one of the preferred standards for fit patients, SLOG represents a biologically and clinically rational alternative that may be further refined for selected populations and treatment settings.
The full article is available in the European Journal of Cancer.