The MATTERHORN study (NCT04592913), presented by Dr. Salah-Eddin Al-Batran from Frankfurt am Main, Germany, at ESMO GI 2025, evaluated the combination of perioperative durvalumab (D) with FLOT chemotherapy versus placebo plus FLOT in patients with resectable gastric or gastroesophageal junction (G/GEJ) adenocarcinoma. This Phase 3, double-blind, global study aimed to determine if adding durvalumab, a PD-L1 inhibitor, to the FLOT regimen could improve treatment outcomes in this patient population.
The study’s primary endpoint was event-free survival (EFS), with overall survival (OS) as a key secondary endpoint. Additionally, patient-reported outcomes (PROs) were assessed using the EORTC QLQ-C30 questionnaire to evaluate quality of life (QoL) changes and treatment tolerability. Dr. Al-Batran’s presentation highlighted how the combination of durvalumab and FLOT may become a potential new standard of care in the perioperative treatment of resectable G/GEJ adenocarcinoma.
Background
The MATTERHORN study addresses a critical need for improved therapies in patients with resectable G/GEJ adenocarcinoma, a disease that often presents with poor prognosis despite surgical resection. Traditionally, chemotherapy regimens like FLOT (fluorouracil, leucovorin, oxaliplatin, and docetaxel) have been used in both neoadjuvant and adjuvant settings, but they have not yielded optimal outcomes for all patients.
The addition of immune checkpoint inhibitors, such as durvalumab, which inhibits PD-L1 to enhance the immune system’s ability to target tumor cells, has the potential to improve clinical outcomes. The MATTERHORN study thus sought to explore whether combining durvalumab with FLOT would provide superior event-free survival compared to the standard FLOT regimen alone.
Methods
In this global, Phase 3, double-blind study, patients with resectable, untreated G/GEJ adenocarcinoma were randomized in a 1:1 ratio to receive either durvalumab (1500 mg) or placebo, both administered every 4 weeks (Q4W) in combination with FLOT chemotherapy (Q2W) for four cycles. The first two cycles of FLOT were administered preoperatively (neoadjuvant), followed by two cycles postoperatively (adjuvant). After surgery, patients continued receiving durvalumab or placebo monotherapy for an additional 10 cycles.
The co-primary endpoints included EFS, defined as the time from randomization to progression, recurrence, or death, and OS, as a key secondary endpoint. Additionally, PROs were assessed by evaluating the time to deterioration (TTD) of global health status, physical functioning, and role functioning using the EORTC QLQ-C30 questionnaire. Safety was also a key focus, with adverse events (AEs) monitored throughout the study.
Results
The results of the MATTERHORN study provide valuable insights into the efficacy and safety of perioperative durvalumab in combination with FLOT chemotherapy for patients with resectable gastric or gastroesophageal junction (G/GEJ) adenocarcinoma. With event-free survival (EFS) as the primary endpoint, the study aimed to assess whether adding durvalumab to the FLOT regimen could improve outcomes compared to placebo plus FLOT.
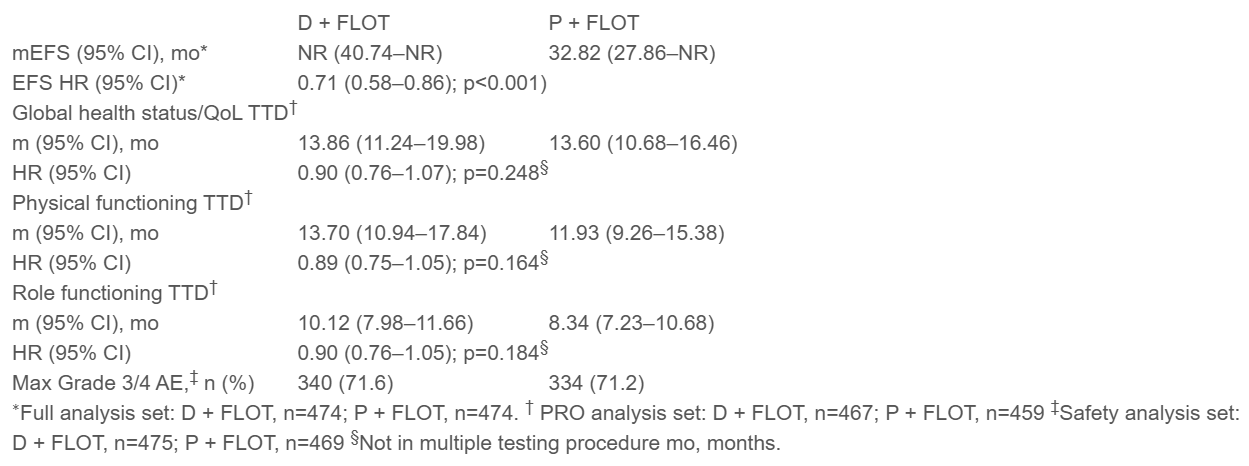
- Event-Free Survival (EFS): The addition of durvalumab to FLOT demonstrated a statistically significant improvement in EFS compared to placebo plus FLOT. The hazard ratio (HR) for EFS was 0.71 (95% CI, 0.58–0.86; p<0.001), with median EFS not yet reached for the durvalumab group versus 32.82 months for the placebo group.
- Overall Survival (OS): At the time of analysis, median OS was also improved in the durvalumab group, although not statistically significant. The median OS was not reached for the durvalumab group, while the placebo + FLOT group had a median OS of 47.21 months (HR, 0.78; 95% CI, 0.62–0.97; p=0.025), suggesting that adding durvalumab may improve long-term survival, with further analysis needed at the final data cut-off.
- Quality of Life (QoL): There were no significant differences between the durvalumab + FLOT and placebo + FLOT groups in terms of time to deterioration (TTD) for global health status or physical functioning. The HR for global health status was 0.90 (95% CI, 0.76–1.07; p=0.248), and for physical functioning, it was 0.89 (95% CI, 0.75–1.05; p=0.164). Both groups showed similar QoL outcomes over time, indicating that the addition of durvalumab did not negatively impact patients’ overall quality of life.
- Adverse Events (AEs): The safety profile of durvalumab + FLOT was generally manageable, with no significant differences in the rates of Grade 3/4 AEs between the two groups. A total of 71.6% of patients in the durvalumab group experienced any Grade 3/4 AEs compared to 71.2% in the placebo group. Immune-mediated AEs (imAEs) were reported in 25.5% of patients receiving durvalumab, compared to 34.1% in the placebo group. These rates were consistent with the expected safety profiles for immune checkpoint inhibitors in combination with chemotherapy.
Conclusions
The MATTERHORN study demonstrated that the addition of durvalumab to the FLOT chemotherapy regimen significantly improves event-free survival (EFS) in patients with resectable G/GEJ adenocarcinoma compared to placebo plus FLOT. Although overall survival (OS) results showed a trend favoring durvalumab, further follow-up is needed to confirm long-term survival benefits. No significant differences in quality of life measures were observed, suggesting that the addition of durvalumab does not negatively affect patient well-being.
The safety profile of durvalumab + FLOT was tolerable, with no new safety signals identified. These findings support the potential for durvalumab + FLOT to become a new global standard of care for the perioperative treatment of resectable G/GEJ adenocarcinoma, offering hope for improved outcomes in this challenging patient population.
You can read the full abstract here


