The ESMO 2025 Congress brings major advances in liver and pancreatic cancer, featuring pivotal trials in hepatocellular carcinoma (HCC), biliary tract cancers (BTC), and pancreatic ductal adenocarcinoma (PDAC). Across these diseases, studies explore immunotherapy, targeted agents, and optimized treatment sequencing aimed at improving survival and expanding curative potential. From next-generation immune combinations in HCC to precision and resistance-overcoming approaches in BTC and breakthrough KRAS inhibition in PDAC, ESMO 2025 signals a turning point toward more personalized, effective care in gastrointestinal oncology.
Hepatocellular Carcinoma Trials
Together, these hepatocellular carcinoma trials illustrate the rapid evolution of systemic therapy in liver cancer. Building on the success of atezolizumab–bevacizumab, the field is moving toward intensified immunotherapy combinations, exploration of earlier-stage use, and integration with surgery. From PD-1/VEGF dual blockade to TIGIT-targeted triplets and systemic therapy replacing or preceding locoregional approaches, ESMO 2025 is set to redefine treatment algorithms across the disease continuum — from unresectable to potentially curable stages.
CS1003-305 Trial: Nofazinlimab + Lenvatinib in First-Line Unresectable HCC
Abstract: LBA52
Presenter: Jia Fan (Shanghai, China)
Trial Type: Phase III, randomized, double-blind
Session: Mini Oral
This phase 3 trial evaluates nofazinlimab, an anti–PD-1 antibody, in combination with lenvatinib versus placebo plus lenvatinib in patients with unresectable or metastatic HCC. The goal is to determine whether PD-1 inhibition enhances outcomes beyond the established VEGF/FGF-targeted standard.
Why it matters: If positive, this trial could expand the first-line immunotherapy options in HCC, challenging the atezolizumab–bevacizumab backbone.
IMbrave152/SKYSCRAPER-14 trial: Tiragolumab + Atezolizumab + Bevacizumab in HCC
Abstract: LBA50
Presenter: Richard S. Finn (Los Angeles, USA)
Trial Type: Phase III
Session: Proffered Paper
This global trial tests PD-L1/ TIGIT dual blockade (tiragolumab + atezolizumab) plus bevacizumab versus the established atezolizumab–bevacizumab regimen in first-line unresectable/metastatic HCC. Tiragolumab blocks TIGIT, a co-inhibitory receptor that suppresses T-cell and NK-cell activity, while atezolizumab restores T-cell function through PD-L1 inhibition. Bevacizumab targets VEGF, improving vascular normalization and immune infiltration.
Findings from the earlier MORPHEUS-Liver phase 1b–2 trial, published in The Lancet Oncology in 2025, showed that adding tiragolumab to atezolizumab + bevacizumab produced a higher objective response rate (43% vs 11%) in patients with previously untreated, unresectable hepatocellular carcinoma. The triplet combination was generally well tolerated, with mainly low-grade pruritus, arthralgia, and diarrhea, and no new safety signals identified.
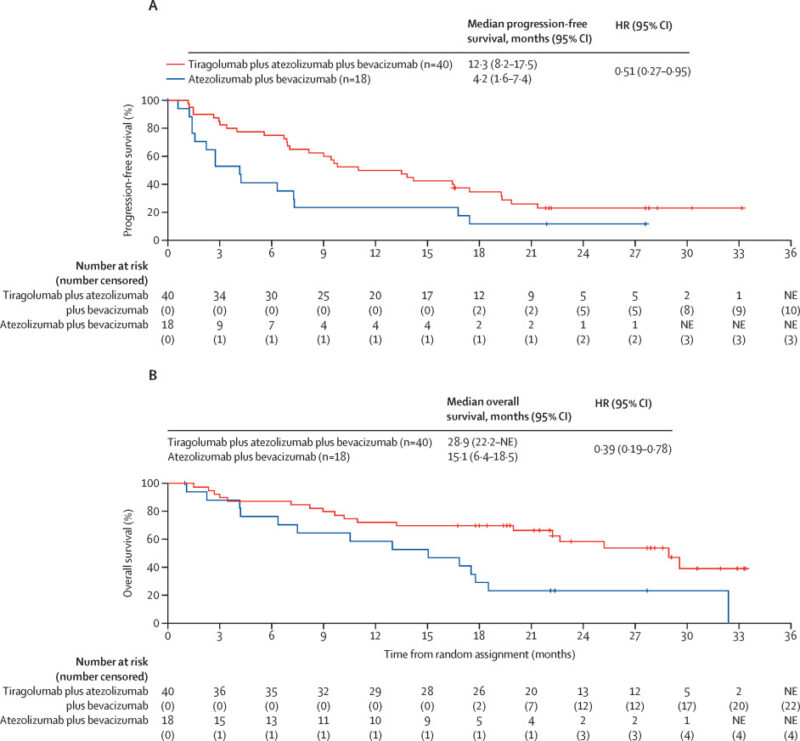
Why it matters: Could define whether TIGIT inhibition further improves survival beyond the current IO-VEGF backbone.
IKF-035/ABC-HCC trial: Atezo/Bev vs TACE in Intermediate HCC
Abstract: LBA51
Presenter: Peter R. Galle (Mainz, Germany)
Trial Type: Phase IIIb
Session: Mini Oral
The ABC-HCC (IKF-035) trial is a randomized, multicenter phase IIIb study comparing atezolizumab plus bevacizumab with transarterial chemoembolization (TACE) in patients with intermediate-stage hepatocellular carcinoma (HCC) exceeding the Milan criteria and without curative options. As published in Journal of Clinical Oncology (ASCO GI 2022), the study aims to determine whether systemic immunotherapy can outperform locoregional therapy in this population. The primary endpoint is time to failure of treatment strategy, with secondary endpoints including overall survival, progression-free survival, and quality of life.
Why it matters: This first head-to-head comparison between immunotherapy and TACE could redefine management of intermediate-stage HCC, marking a paradigm shift toward systemic therapy in earlier disease stages.
Bile Duct Cancer Trials
Advances in cholangiocarcinoma and biliary tract cancers (BTC) are redefining treatment options for these historically hard-to-treat diseases. At ESMO 2025, studies of immunotherapy, targeted therapy, and neoadjuvant strategies—including toripalimab + lenvatinib + GEMOX, durvalumab + tremelimumab, and tinengotinib—highlight the growing shift toward precision, immune-based, and resistance-overcoming approaches in bile duct cancer.
Toripalimab + Lenvatinib + GEMOX in Resectable Intrahepatic Cholangiocarcinoma
Abstract: LBA11
Presenter: Guoming Shi (Shanghai, China)
Trial Type: Phase II–III
Session: Mini Oral
Earlier findings from a Nature Cell Discovery phase 2 study (Shi et al., 2023) showed that the combination of toripalimab, lenvatinib, and GEMOX achieved an impressive 80% objective response rate and a median overall survival of 22.5 months in advanced intrahepatic cholangiocarcinoma, with manageable grade ≥3 toxicities such as neutropenia and leukocytopenia. Responses were particularly pronounced in patients with DNA damage response (DDR) mutations.
Building on this efficacy signal, the current phase II–III neoadjuvant trial investigates whether this immuno-targeted triplet can improve pathological responses and long-term outcomes in high-risk, resectable ICC.
Why it matters: If positive, this study could pioneer an immuno-targeted neoadjuvant approach for cholangiocarcinoma, a disease with historically poor surgical outcomes and limited systemic options.
Read more about Toripalimab (Loqtorzi): Uses in Cancer, Side Effects, Dosage, Expectations on OncoDaily.
IMMUNOBIL Trial: Durvalumab + Tremelimumab in BTC
Abstract: 80MO
Presenter: Matthieu Delaye (Paris, France)
Trial Type: Phase II (PRODIGE-57)
Session: Mini Oral
The IMMUNOBIL GERCOR D18-1 PRODIGE-57 study evaluated dual immune checkpoint blockade with durvalumab (anti–PD-L1) and tremelimumab (anti–CTLA-4) in patients with advanced biliary tract cancer (BTC) who had progressed after platinum-based chemotherapy.
Findings, published in The Journal of Clinical Oncology in 2022, showed a 6-month overall survival rate of 59%, with a median OS of 8.0 months and an objective response rate of 9.7%. Durable responses were seen in patients achieving disease control, and the safety profile was manageable, with fatigue and diarrhea as the most common grade 3–4 treatment-related adverse events.
Why it matters: Although exploratory, the study met its predefined efficacy threshold, supporting durvalumab + tremelimumab as a promising late-line option for BTC and paving the way for further evaluation of dual checkpoint blockade in this setting.
Tinengotinib in FGFR-Refractory Cholangiocarcinoma
Abstract: 81MO
Presenter: Milind Javle (Houston, USA)
Trial Type: Pooled analysis
Session: Mini Oral
Tinengotinib is a next-generation multikinase inhibitor designed to overcome acquired resistance to first-generation FGFR inhibitors in FGFR2-altered cholangiocarcinoma. Early-phase studies showed promising activity in heavily pretreated patients with FGFR inhibitor–refractory disease.
The FIRST-308 phase III trial, published in The Journal of Clinical Oncology in 2024, is comparing oral tinengotinib versus physician’s choice of chemotherapy (FOLFOX or FOLFIRI) in patients with FGFR-altered, chemotherapy- and FGFRi-refractory/relapsed CCA. The primary endpoint is progression-free survival, with overall survival and quality of life as key secondary endpoints.
Why it matters: Tinengotinib may offer a new therapeutic avenue for patients who relapse after FGFR-targeted therapy, addressing one of the most challenging resistance settings in advanced cholangiocarcinoma.
Pancreatic Cancer Trials
Pancreatic ductal adenocarcinoma (PDAC) remains one of the most challenging malignancies, characterized by late diagnosis, rapid progression, and limited curative options. The 2025 ESMO Congress spotlights emerging strategies aimed at improving both surgical and systemic outcomes—from refining perioperative chemotherapy approaches to advancing molecularly targeted therapies. Collectively, these developments reflect a growing emphasis on personalized, biology-driven treatment in a disease long resistant to therapeutic innovation.
Short- vs Long-Course Neoadjuvant Chemotherapy in Resectable PDAC
Abstract: LBA85
Presenter: Michele Reni (Milan, Italy)
Trial Type: Phase III
Session: Mini Oral
This randomized phase 3 study compares short-course versus long-course preoperative chemotherapy in patients with stage I–III pancreatic ductal adenocarcinoma (PDAC), aiming to define the optimal duration of neoadjuvant therapy that maximizes surgical outcomes while minimizing toxicity.
Why it matters: The trial could clarify how much preoperative treatment is enough to improve resectability and long-term survival without compromising tolerance.
GFH375 in KRAS G12D-Mutant PDAC
Abstract: LBA84
Presenter: Aiping Zhou (Beijing, China)
Trial Type: Phase II/III
Session: Proffered Paper
GFH375 is a highly selective oral KRAS G12D inhibitor targeting both active (GTP-bound) and inactive (GDP-bound) forms of the protein. In a first-in-human phase I/II study presented at the 2025 ASCO Annual Meeting and published in JCO, GFH375 showed encouraging safety and efficacy across multiple tumor types, including pancreatic ductal adenocarcinoma (PDAC).
Among 32 patients treated, including 11 with PDAC, no dose-limiting toxicities were observed. The most common treatment-related adverse events were mild gastrointestinal effects. Across evaluable patients, the objective response rate was 27%, and the disease control rate reached 86%. Notably, all PDAC patients showed tumor shrinkage, including three partial responses.
Why it matters: These early results support GFH375 as a promising first-in-class KRAS G12D inhibitor, potentially unlocking a long-sought therapeutic target in pancreatic cancer.
Takeaway
These 11 trials underscore how ESMO 2025 is reshaping liver and pancreatic cancer care. In HCC, systemic immunotherapy continues to move earlier, challenging locoregional standards and exploring novel backbones like TIGIT blockade. In biliary cancers, IO and next-generation targeted agents expand options in both neoadjuvant and refractory settings. In pancreatic cancer, strategies range from KRAS targeting and optimal chemotherapy sequencing to innovative platforms like oncolytic viruses and ADCs. Together, they signal a shift toward precision, combination-based, and multimodal strategies in GI oncology.

Read about Top Upper GI Cancer Trials to Watch at ESMO 2025 on OncoDaily


