The ESMO 2025 Congress in October is expected to deliver practice-shaping data across upper gastrointestinal (GI) cancers, including gastric, esophageal, pancreatic, and hepatocellular carcinoma (HCC). From perioperative immunotherapy and novel targeted agents to oncolytic virotherapy and multimodal treatment strategies, several highly anticipated studies are poised to make headlines.
This overview highlights the most clinically relevant upper GI cancer trials to be presented at ESMO 2025, with potential to inform practice and shape future standards of care.
Pancreatic Cancer Trials
Pancreatic ductal adenocarcinoma (PDAC) remains one of the deadliest cancers, but several promising strategies are emerging. Key trials include telisotuzumab adizutecan (Temab-A), a c-Met–targeting antibody-drug conjugate; HRS-4642, a selective KRAS-G12D inhibitor combined with chemotherapy; and VIRAGE, testing the oncolytic virus VCN-01 with nab-paclitaxel and gemcitabine. Together, these studies explore precision targeting and immune activation to improve first-line and metastatic PDAC outcomes.
Phase 1 Basket Study of Telisotuzumab Adizutecan (ABBV-400; Temab-A) in Pancreatic Ductal Adenocarcinoma
Abstract: 2214MO
Presenter: James J. Harding (New York, United States of America)
Trial Type: Phase I Basket Study
Session: Mini Oral Session – GI tumours, upper digestive
Telisotuzumab adizutecan (ABBV-400; Temab-A) is a c-Met–targeting antibody-drug conjugate being evaluated across multiple solid tumors. This basket study includes a cohort of patients with pancreatic ductal adenocarcinoma, aiming to assess the safety profile and preliminary antitumor activity of Temab-A in this difficult-to-treat population.
Why It Matters? This update is important because it explores c-Met as a therapeutic target in PDAC — a population with few actionable drivers — and could open the door for ADC development in this difficult setting.
HRS-4642 Combined with Gemcitabine and Nab-paclitaxel in KRAS-G12D Mutant Advanced Pancreatic Cancer: Phase 1b/2 Study
Abstract: 2215MO
Presenter: Liwei Wang (Shanghai, China)
Trial Type: Phase 1b/2
Session: Mini Oral Session – GI tumours, upper digestive
HRS-4642 is a selective KRAS-G12D inhibitor designed to target one of the most common oncogenic drivers in pancreatic ductal adenocarcinoma (PDAC). This phase 1b/2 study evaluates HRS-4642 in combination with gemcitabine and nab-paclitaxel in patients with KRAS-G12D mutant advanced PDAC.
Why It Matters? These results will show whether directly targeting KRAS-G12D, the most common mutation in PDAC, can enhance first-line chemotherapy efficacy and offer a precision-medicine approach for a large subgroup of patients.
VIRAGE Trial: Nab-Paclitaxel and Gemcitabine ± Intravenous VCN-01 in Metastatic Pancreatic Cancer (mPDAC)
Abstract: 2216MO
Presenter: Rocio Garcia-Carbonero (Madrid, Spain)
Trial Type: Phase IIb, Randomized, Open-Label
Session: Mini Oral Session – GI tumours, upper digestive
The VIRAGE trial is a randomized phase IIb study evaluating the addition of VCN-01, an oncolytic adenovirus, to standard nab-paclitaxel and gemcitabine in patients with metastatic pancreatic ductal adenocarcinoma. VCN-01 is designed to selectively replicate in tumor cells and enhance immune activation within the tumor microenvironment.
Why It Matters? VIRAGE will provide the first randomized data on systemic oncolytic virotherapy in PDAC, and will clarify whether adding systemic virotherapy to chemotherapy can improve outcomes and support its use in first-line treatment.
Gastric and Esophageal Cancers Trials
Major studies in esophageal and gastric cancer are advancing immunotherapy and multimodality treatment. SKYSCRAPER-07 will report atezolizumab ± tiragolumab after chemoradiotherapy in unresectable ESCC, PHERFLOT/IKF-053 will present interim results of pembrolizumab + trastuzumab with perioperative FLOT in HER2-positive esophagogastric cancer, and PERISCOPE II will share randomized data on gastrectomy + CRS/HIPEC for gastric cancer with limited peritoneal spread. These studies could shift standards for immunotherapy and surgery in upper GI oncology.
SKYSCRAPER-07: Atezolizumab ± Tiragolumab After Definitive Chemoradiotherapy in Unresectable Esophageal Squamous Cell Carcinoma (ESCC)
Abstract: 2094O
Presenter: Ian Chau (Sutton, United Kingdom)
Trial Type: Phase III, Randomized
Session: Proffered Paper Session – GI tumours, upper digestive
SKYSCRAPER-07 is a global, randomized, phase III trial evaluating maintenance atezolizumab with or without tiragolumab (an anti-TIGIT antibody) in patients with unresectable ESCC who have not progressed following definitive concurrent chemoradiotherapy (dCRT). This study builds on the success of PD-L1 blockade in the locally advanced setting and explores whether dual ICI therapy can improve survival by further enhancing antitumor immune activity.
Why It Matters? This trial could establish TIGIT inhibition as a new consolidative option after chemoradiotherapy in unresectable ESCC, potentially deepening and prolonging immune control of the disease.
PHERFLOT/IKF-053: Pembrolizumab + Trastuzumab + FLOT in Perioperative HER2-Positive Localized Esophagogastric Adenocarcinoma
Abstract: 2095MO
Presenter: Joseph Tintelnot (Hamburg, Germany)
Trial Type: Phase II, Interim Analysis
Session: Mini Oral Session – GI tumours, upper digestive
The PHERFLOT/IKF-053 trial (AIO STO 0321) is a phase II, multicenter study evaluating the addition of pembrolizumab and trastuzumab to perioperative FLOT chemotherapy in patients with HER2-positive localized esophagogastric adenocarcinoma. The trial aims to determine whether combining dual HER2- and PD-1–targeted therapy with standard perioperative chemotherapy can increase pathological complete response rates and improve survival outcomes.
Why It Matters? This study could support integrating PD-1 blockade and HER2-targeted therapy into perioperative FLOT, raising pCR rates and shifting the standard of care in HER2-positive localized disease.
PERISCOPE II Trial: Systemic Therapy + Gastrectomy + CRS/HIPEC vs Systemic Therapy Alone in Gastric Cancer with Limited Peritoneal Dissemination
Abstract: 2096MO
Presenter: Judith S. Quik (Amsterdam, Netherlands)
Trial Type: Phase III, Randomized
Session: Mini Oral Session – GI tumours, upper digestive
PERISCOPE II is a randomized, multicenter phase III trial investigating whether combining systemic therapy with gastrectomy and cytoreductive surgery (CRS) plus hyperthermic intraperitoneal chemotherapy (HIPEC) improves outcomes compared to systemic therapy alone in patients with gastric cancer and limited peritoneal dissemination. This high-risk subgroup has historically had poor survival, and evidence for aggressive local treatment approaches has been limited.
Earlier, preliminary data from PERISCOPE II were published in the European Journal of Surgical Oncology (Vol. 49, Issue 2, E40, Feb 2023). This multicentre randomized phase III trial is comparing systemic chemotherapy alone with gastrectomy, cytoreductive surgery (CRS), and hyperthermic intraperitoneal chemotherapy (HIPEC) in gastric cancer patients with limited peritoneal metastases (PCI < 7).
Recruitment began in 2017 across multiple European centers, targeting 226 patients. The primary endpoint is overall survival. These results are expected to provide the first randomized evidence on whether surgery plus HIPEC can improve survival compared with chemotherapy alone in this high-risk group.
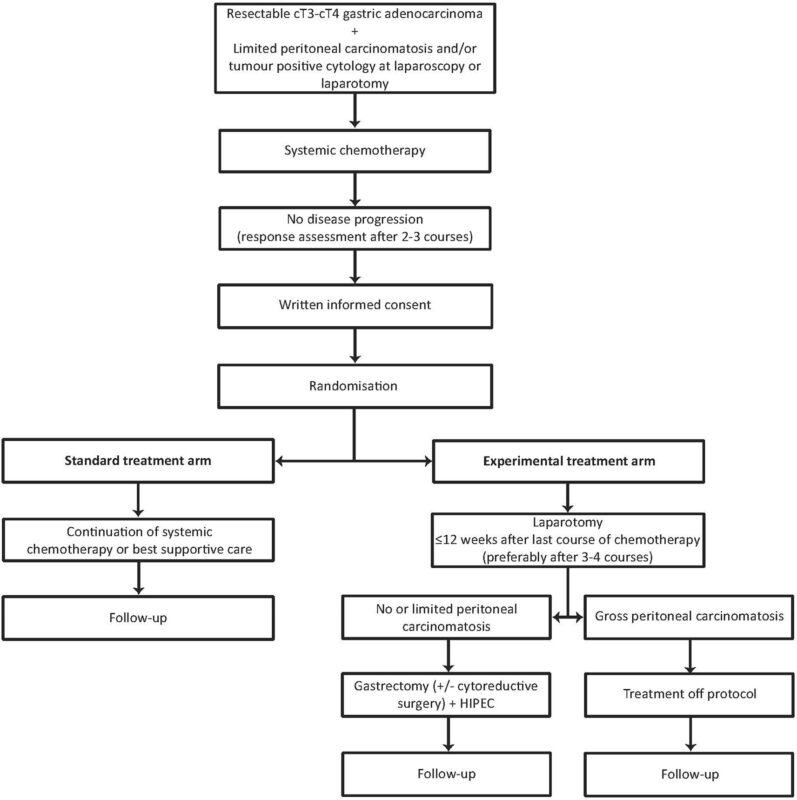
Why It Matters? Results will address a decades-long debate on whether aggressive locoregional therapy with gastrectomy, CRS, and HIPEC offers a survival benefit over chemotherapy alone for patients with limited peritoneal disease.
Hepatocellular Carcinoma Trials
Key trials in HCC are exploring new strategies across all stages of disease. TALENTop compares liver resection after atezo/bev response with continued systemic therapy, CARES-009 evaluates perioperative camrelizumab plus rivoceranib to reduce recurrence after surgery, and TRIPLET HCC tests the addition of ipilimumab to atezo/bev in first-line unresectable HCC. Together, these studies could shift standards for surgery, perioperative therapy, and systemic treatment in HCC.
TALENTop Trial: Liver Resection vs Continued Atezolizumab + Bevacizumab After Response in Locally Advanced HCC
Abstract: 1469MO
Presenter: Hui-Chuan Sun (Shanghai, China)
Trial Type: Phase III, Randomized, Open-Label
Session: Mini Oral Session – GI tumours, hepatobiliary
TALENTop is a multicenter, open-label, randomized phase III trial evaluating the role of surgery after systemic therapy in hepatocellular carcinoma (HCC). Patients with initially unresectable, locally advanced HCC who achieved tumor downstaging on atezolizumab plus bevacizumab (atezo/bev) were randomized to either undergo liver resection or continue atezo/bev treatment. This trial addresses a key clinical question: whether conversion surgery following immunotherapy-based systemic treatment can improve long-term survival compared to continuing immunotherapy alone.
Earlier data from the Chinese TALENTop study (NCT04649489) were published as a “Publication Only” abstract at the 2024 ASCO Annual Meeting. This multicenter randomized phase III trial evaluates whether liver resection after response to induction atezolizumab + bevacizumab improves outcomes compared with continued systemic therapy in patients with hepatocellular carcinoma (HCC) and macrovascular invasion.
Among 296 patients enrolled in the induction phase, 175 were not randomized—mostly due to disease progression—and were followed for survival outcomes. These patients had a median overall survival of 13.8 months, with better outcomes observed in those receiving combined systemic and locoregional therapy (mOS 16.2 months). Upcoming results will reveal whether conversion surgery can further extend survival in patients who respond to immunotherapy-based treatment.
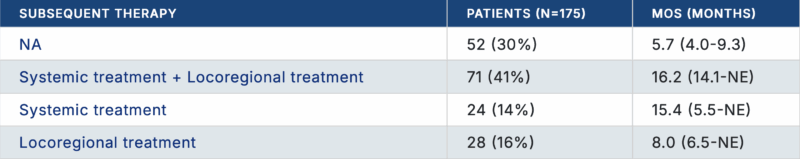
Why It Matters? This trial will determine if conversion surgery after immunotherapy truly improves survival, potentially establishing a new standard of care for patients who respond to atezo/bev.
CARES-009 Trial: Perioperative Camrelizumab + Rivoceranib in Resectable Hepatocellular Carcinoma (HCC)
Abstract: 1470MO
Presenter: Jian Zhou (Shanghai, China)
Trial Type: Phase III, Randomized, Multicenter
Session: Mini Oral Session – GI tumours, hepatobiliary
CARES-009 is a randomized, multicenter phase III trial evaluating the combination of camrelizumab and rivoceranib as perioperative therapy for patients with resectable HCC. Camrelizumab is a PD-1 immune checkpoint inhibitor that reactivates T cells to attack cancer. Rivoceranib is an oral VEGFR2 tyrosine kinase inhibitor that blocks tumor angiogenesis. Together, they pair immune activation with anti-angiogenic therapy, a combination shown to enhance responses in several cancers. The study investigates whether adding immunotherapy and targeted anti-angiogenic therapy to surgery can reduce recurrence risk and improve long-term survival compared to surgery alone.
Why It Matters? CARES-009 will show whether perioperative PD-1 + VEGFR2 blockade reduces recurrence and may support its use as part of curative treatment.
PRODIGE 81/FFCD 2101 – TRIPLET HCC: Ipilimumab + Atezolizumab + Bevacizumab in First-Line Unresectable HCC
Abstract: 1471MO
Presenter: Philippe Merle (Lyon, France)
Trial Type: Phase II/III, Randomized
Session: Mini Oral Session – GI tumours, hepatobiliary
TRIPLET HCC (PRODIGE 81/FFCD 2101) is a randomized trial evaluating whether adding ipilimumab (CTLA-4 inhibitor) to the established atezolizumab + bevacizumab regimen can further improve outcomes in patients with unresectable hepatocellular carcinoma (uHCC) receiving first-line systemic therapy. The study aims to enhance immune activation and deepen responses beyond those seen with dual PD-L1/VEGF inhibition.
Preliminary safety results from the French PRODIGE 81/FFCD 2101 TRIPLET-HCC trial were published in the Journal of Clinical Oncology (Vol. 42, Suppl 16, 2024). This randomized phase II/III study evaluates the addition of ipilimumab to atezolizumab + bevacizumab as first-line therapy for HCC.
In the safety run-in of the first 15 patients on triple therapy, most completed induction with manageable immune-related toxicities and no unexpected safety concerns. The independent monitoring board approved continuation of the trial, paving the way for upcoming efficacy readouts on response and survival.
Why It Matters? This study will reveal whether triple therapy with ipilimumab, atezolizumab, and bevacizumab delivers deeper and more durable responses than the current atezo/bev standard, which could reshape first-line systemic treatment.

Read more about Top Colorectal Cancer Trials to Watch at ESMO 2025 on OncoDaily.


