The phase 3 CheckMate 8HW study, presented at the ASCO 2025 annual meeting, delivers compelling evidence that dual immunotherapy with nivolumab (NIVO) plus ipilimumab (IPI) offers unprecedented and sustained clinical benefit for patients with microsatellite instability-high (MSI-H) or mismatch repair-deficient (dMMR) metastatic colorectal cancer (mCRC).
Study Design and Background
The global, multicenter CheckMate 8HW trial (NCT04008030) enrolled patients with locally confirmed MSI-H/dMMR mCRC, then centrally re-confirmed status using IHC and PCR. Patients were randomized to receive first-line (1L) NIVO + IPI, NIVO alone, or standard chemotherapy. A key exploratory endpoint was PFS2—the time from randomization to progression after subsequent systemic therapy or death—offering a comprehensive view of long-term benefit beyond initial treatment.
Results: NIVO + IPI Delivers Durable Benefit
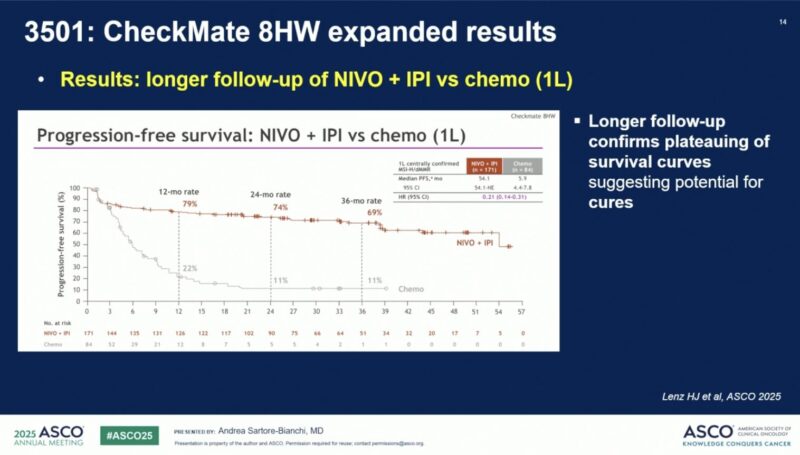
Among all randomized 1L patients with centrally confirmed MSI-H/dMMR mCRC, NIVO + IPI achieved a median progression-free survival (PFS) of 54.1 months (95% CI, 54.1–NE), compared to 5.9 months (95% CI, 4.4–7.8) for chemotherapy (HR 0.21, 95% CI, 0.14–0.31), a nearly fivefold improvement. Median PFS2 was also notably longer with immunotherapy (NR vs 30.3 months; HR 0.28).
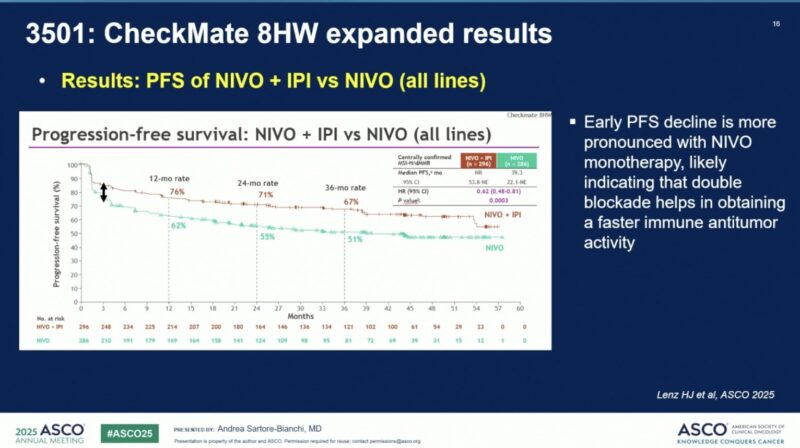
When considering all lines of therapy, NIVO + IPI continued to outperform NIVO alone, achieving median PFS not reached (95% CI, 53.8–NE) versus 39.3 months (95% CI, 22.1–NE) for NIVO monotherapy (HR 0.62, P = 0.0003). The superior benefit of dual immunotherapy was further supported by PFS2 (HR 0.57).
Importantly, fewer patients needed subsequent therapy after 1L NIVO + IPI (16%) versus chemotherapy (73%), and fewer required further immunotherapy. No new safety signals were observed, with grade 3/4 treatment-related adverse events in 22% (NIVO + IPI) and 14% (NIVO) of treated patients.
Clinical Implications
The CheckMate 8HW data confirm that NIVO + IPI provides robust and sustained clinical benefit compared to both chemotherapy and single-agent immunotherapy in MSI-H/dMMR mCRC. The results are particularly striking in first-line patients, with more than four years median PFS and durable responses observed. PFS2 outcomes reinforce the benefit of initial dual immunotherapy, even after subsequent therapies.
Given these findings and a manageable safety profile, NIVO + IPI should now be considered a standard of care for patients with MSI-H/dMMR mCRC.
What They’re Saying: Reactions toCheckMate 8HW Trial at ASCO 2025
Ryan Huey, MD, MS GI Medical Oncologist, from MDAnderson Cancer Center, shared on X
Dr. Lenz with update to CheckMate 8HW (nivo/ipi vs nivo vs chemo in MSI-H CRC). Median duration of tx 20.5 vs 16.4 vs 5.1 months. Median PFS2 HR 0.28 (only 71% of pts who started w/ chemo got IO next?)
Dr Amol Akhade: Oncologist from Mumbai shared on X
CheckMate 8HW ASCO 2025 Update
1L MSI-H/dMMR mCRC:
NIVO + IPI vs Chemo
Median PFS: 54.1 vs 5.9 mo
HR: 0.21
3-yr PFS: 69% vs 11%
Plateauing curve = durable control, potential cure
NIVO + IPI > NIVO alone too
HR: 0.62 | 3-yr PFS: 67% vs 51%
Dual IO = deeper, faster response
New SOC for 1L MSI-H CRC?
Arndt Vogel, Clinician-Scientist, ESMO Ambassador, focussed on Liver Cancer & Precision Oncology, from Toronto General Hospital/Princess Margaret Cancer Center shared on X
Precision Oncology in the First-Line Setting of Colorectal Cancer ASCO 2025 Excellent discussion by Andrea Sartore-Bianchi BREAKWATER and CHECKMATE 8HW are practice changing
Takeaway Messages from the CheckMate 8HW Trial
Robust Efficacy: Nivolumab plus ipilimumab (NIVO + IPI) demonstrated substantial superiority over chemotherapy and single-agent nivolumab, significantly extending progression-free survival (PFS) and second progression-free survival (PFS2) in patients with MSI-H/dMMR metastatic colorectal cancer (mCRC).
Unprecedented Survival Benefit: The dual immunotherapy approach achieved a remarkable median PFS of 54.1 months compared to only 5.9 months with chemotherapy, emphasizing its transformative potential for first-line treatment.
Durable Responses: Superior results were sustained even after subsequent lines of therapy (PFS2), reflecting durable clinical benefit and prolonged disease control, with a hazard ratio (HR) of 0.28 versus chemotherapy.
Reduction in Subsequent Therapy Need: Only 16% of patients receiving first-line NIVO + IPI required subsequent therapies, compared to 73% after chemotherapy, underscoring improved frontline efficacy.
Consistent Benefit Across Lines of Therapy: When assessed across all therapy lines, NIVO + IPI consistently outperformed NIVO alone, achieving a significantly better median PFS (HR 0.62) and improved PFS2 (HR 0.57).
Favorable Safety Profile: The combination maintained an acceptable safety profile, with manageable rates of grade 3/4 adverse events, reinforcing its viability as a frontline treatment choice.
Clinical Practice Impact: These compelling results strongly advocate for the adoption of dual immunotherapy (NIVO + IPI) as the new standard of care for patients with MSI-H/dMMR metastatic colorectal cancer, significantly altering clinical treatment strategies.
This pivotal study marks a new benchmark in metastatic colorectal cancer treatment, highlighting dual immunotherapy as a transformative approach with long-term benefits.
You Can Watch More on OncoDaily Youtube TV
Written by Armen Gevorgyan, MD


