Ipilimumab and nivolumab combination, On April 8, 2025, twas approved by he U.S. Food and Drug Administration (FDA) for the treatment of adult and pediatric patients aged 12 years and older with unresectable or metastatic microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR) colorectal cancer (CRC). Additionally, the FDA converted the accelerated approval of single-agent nivolumab to regular approval for this patient population.
What is the rationale for FDA approval of Ipilimumab and Nivolumab in dMMR CRC?
The phase 3 CheckMate 8HW trial was conducted to evaluate the efficacy and safety of nivolumab plus ipilimumab versus nivolumab monotherapy or chemotherapy ± targeted therapy in patients with MSI-H/dMMR mCRC. Patients were enrolled across multiple lines of therapy with stratification by tumor location and number of prior treatments. Only patients with centrally confirmed MSI-H/dMMR status were included in the primary efficacy analysis.
This open-label, international randomized trial enrolled 839 patients with unresectable or metastatic MSI-H/dMMR colorectal cancer across 23 countries and 128 sites. Patients were randomized according to prior lines of treatment:
- First-line setting (Part 2): 2:2:1 randomization to nivolumab + ipilimumab, nivolumab monotherapy, or chemotherapy ± targeted therapy.
- Second-line or beyond (Part 1): 1:1 randomization to nivolumab + ipilimumab or nivolumab.
Nivolumab (240 mg) and ipilimumab (1 mg/kg) were administered every 3 weeks for 12 weeks, followed by maintenance nivolumab every 4 weeks. The primary endpoints were progression-free survival (PFS) by blinded independent central review (BICR) in:
- Nivolumab + ipilimumab vs chemotherapy (first-line)
- Nivolumab + ipilimumab vs nivolumab (all lines)
Results
At the August 2024 data cutoff, median follow-up was 47.0 months. A total of 703 patients were treated, including 582 with centrally confirmed MSI-H/dMMR status forming the primary efficacy population (296 on nivolumab + ipilimumab; 286 on nivolumab).
Nivolumab + ipilimumab vs nivolumab:
- Median PFS: Not reached vs 39.3 months
- Hazard Ratio (HR): 0.62 (95% CI: 0.48–0.81; p=0.0003)
- 36-month PFS rates: 68% vs 51%
Nivolumab + ipilimumab vs chemotherapy (first-line):
- Median PFS: 54.1 months vs 5.9 months
- HR: 0.21 (95% CI: 0.14–0.31)
- 3-year PFS rate: 69% vs 11%
Objective Response Rate (ORR)
- Nivolumab + ipilimumab: 71% (30% complete response)
- Nivolumab: 58% (28% complete response)
- Progressive disease as best response: 10% vs 19%, respectively
- Median time to response: 2.8 months for both arms
The CheckMate 8HW trial provides compelling evidence that dual checkpoint blockade with nivolumab plus ipilimumab offers superior clinical benefit compared to nivolumab alone or chemotherapy in patients with MSI-H/dMMR metastatic colorectal cancer. With durable responses, extended progression-free survival, and a manageable toxicity profile, this regimen represents a major therapeutic advancement and reinforces the role of immunotherapy as a cornerstone in the management of this molecularly defined subgroup. The data strongly support the combination’s adoption as a preferred frontline and subsequent therapy for eligible patients.
Here you can read about trials results.
What is Colorectal Cancer ?
Colorectal cancer (CRC) is the third most frequently diagnosed cancer and the second leading cause of cancer-related deaths worldwide. Key risk factors for colon cancer encompass advancing age, family history of colorectal cancer, personal history of colorectal adenomas or ovarian cancer, hereditary conditions like familial adenomatous polyposis (FAP) and Lynch syndrome, chronic inflammatory bowel diseases (e.g., ulcerative colitis or Crohn’s disease), excessive alcohol consumption, cigarette smoking, African American ethnicity, and obesity.
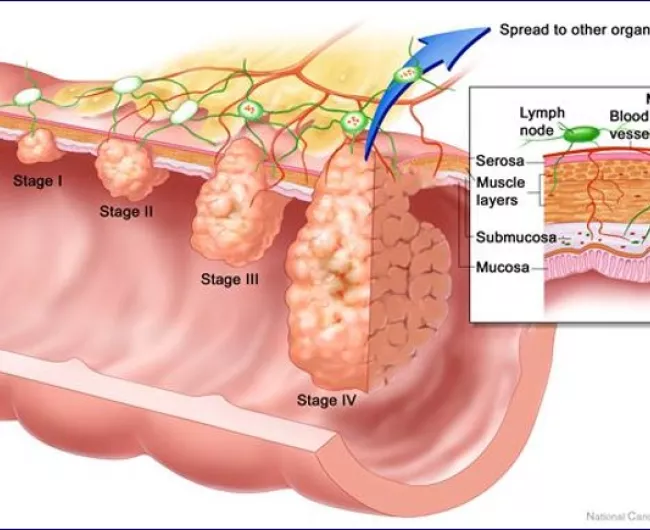
Routine screening is recommended for adults aged 50 and above, particularly those with a first-degree relative diagnosed with colorectal cancer. Early detection through screening is vital due to the disease’s prevalence, the ability to identify high-risk groups, the slow progression of primary lesions, improved survival rates with early-stage detection, and the relative simplicity and accuracy of screening tests.
Patient prognosis is closely linked to the tumor’s penetration depth through the bowel wall, lymph node involvement, and the presence of distant metastases. Additional indicators of a poorer prognosis include bowel obstruction, perforation, and elevated pretreatment serum levels of carcinoembryonic antigen (CEA).
A subset of colorectal cancers, characterized by high microsatellite instability (MSI-H) or deficient mismatch repair (dMMR), tend to have a poorer prognosis and limited response to conventional chemotherapies. Immune checkpoint inhibitors, such as nivolumab and ipilimumab, have emerged as promising treatments by enhancing the body’s immune response against tumor cells.
Read more About Immunotherapy for Colorectal cancer on OncoDaily.

How Ipilimumab and Nivolumab Work
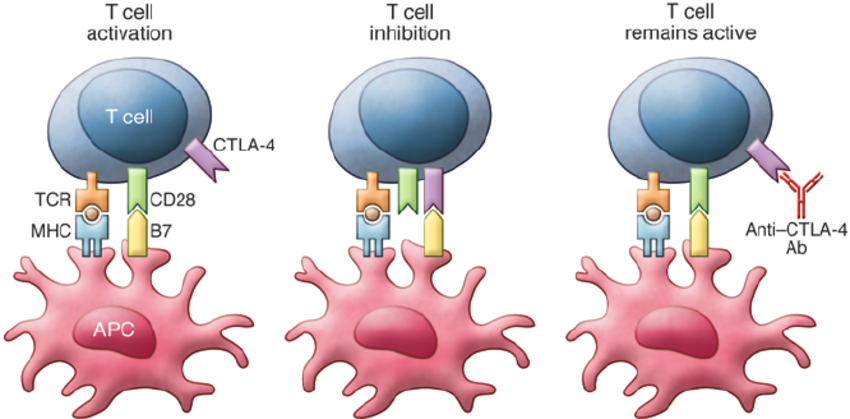
Ipilimumab is a monoclonal antibody that works by enhancing the body’s immune response against cancer cells. Its mechanism of action involves targeting CTLA-4 (cytotoxic T-lymphocyte-associated protein 4), an immune checkpoint receptor expressed on T cells.
Under normal conditions, CTLA-4 functions as a negative regulator of T-cell activation. When T cells are activated by antigen-presenting cells (APCs), CTLA-4 is upregulated and competes with the costimulatory receptor CD28 for binding to B7 molecules (CD80/CD86) on APCs. Binding of CTLA-4 to B7 sends an inhibitory signal that dampens the T-cell response, maintaining immune homeostasis and preventing autoimmunity.
Ipilimumab blocks CTLA-4, thereby inhibiting this negative feedback mechanism. As a result, T-cell activation and proliferation are enhanced, especially cytotoxic T cells, which are capable of recognizing and killing tumor cells. This immune checkpoint blockade effectively amplifies antitumor immunity.
 Nivolumab is a fully human IgG4 monoclonal antibody that functions as an immune checkpoint inhibitor targeting the programmed death-1 (PD-1) receptor on T cells.
Nivolumab is a fully human IgG4 monoclonal antibody that functions as an immune checkpoint inhibitor targeting the programmed death-1 (PD-1) receptor on T cells.
PD-1 is an inhibitory receptor expressed on activated T cells, B cells, and natural killer (NK) cells. Its ligands, PD-L1 and PD-L2, are often expressed on tumor cells and tumor-infiltrating immune cells. When PD-1 binds to PD-L1 or PD-L2, it transmits an inhibitory signal that reduces T-cell proliferation, cytokine production, and cytotoxic activity. This pathway is exploited by tumors to evade immune surveillance by creating an immunosuppressive microenvironment.
Nivolumab binds to PD-1 and prevents its interaction with PD-L1 and PD-L2, thereby releasing the “brakes” on T cells. This blockade restores T-cell function, enhances their proliferation and cytokine release, and reactivates antitumor immune responses.
You Can Read More About Nivolumab (Opdivo) on OncoDaily
Standart of care for patients with unresectable or metastatic MSI-H or dMMR cancer
The standard of care for patients with unresectable or metastatic microsatellite instability-high (MSI-H) or mismatch repair deficient (dMMR) colorectal cancer (CRC) has been redefined by the success of immune checkpoint inhibitors, which offer durable responses and improved outcomes compared to traditional chemotherapy.
Microsatellite instability-high (MSI-H) and mismatch repair-deficient (dMMR) tumors account for approximately 4–7% of metastatic colorectal cancer (mCRC) cases and are generally associated with poor prognosis when treated with chemotherapy alone. While immune checkpoint inhibitors (ICIs) have significantly improved outcomes in this subgroup, notably with pembrolizumab in the KEYNOTE-177 trial, up to 29% of patients still experience primary progression, underscoring an unmet clinical need. Dual ICI therapy, particularly combining anti–PD-1 and anti–CTLA-4 agents, has shown encouraging results in non-randomized settings, prompting further investigation in a randomized phase 3 setting.
For more information click here
You can also check out some new information about MSS colon cancer.
Written by Sona Karamyan, MD


