Esophageal cancer (EC) and gastroesophageal junction cancer (GEJC) remain aggressive malignancies with poor prognosis, even after curative-intent surgery and neoadjuvant chemoradiotherapy (CRT). A significant subset of patients harbor residual pathologic disease post-treatment, placing them at high risk for recurrence and metastasis. The CheckMate 577 trial (NCT02743494), a global, randomized phase 3 study, initially demonstrated that adjuvant nivolumab, an immune checkpoint inhibitor targeting PD-1, significantly improved disease-free survival (DFS) compared with placebo in patients with resected EC/GEJC with residual disease following neoadjuvant CRT and surgery.
At ASCO 2025, investigators reported the final overall survival (OS) analysis, along with updated longer-term follow-up for DFS, distant metastasis-free survival (DMFS), and progression-free survival on subsequent systemic therapy (PFS2), providing a more comprehensive understanding of the clinical benefit and safety of adjuvant nivolumab in this patient population.
Study Design and Methods
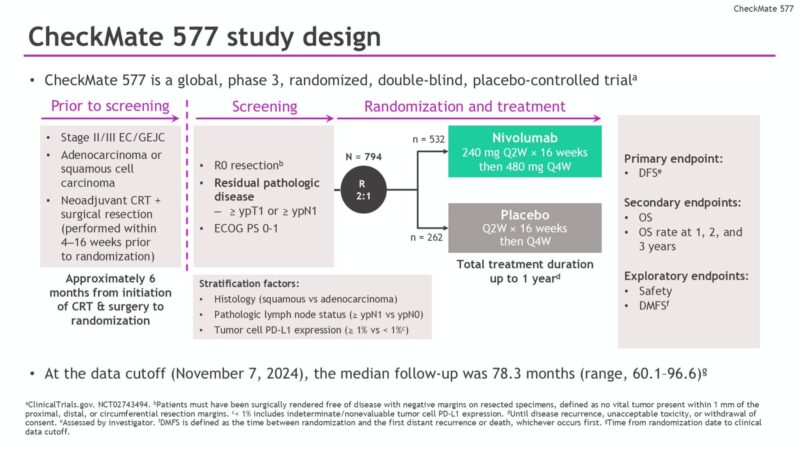
This double-blind, placebo-controlled trial enrolled adults with resected (R0) stage II/III EC or GEJC who had undergone neoadjuvant CRT and had residual pathologic disease confirmed at surgery. Patients were randomized in a 2:1 ratio to receive adjuvant nivolumab (240 mg intravenously every 2 weeks for 16 weeks, followed by 480 mg every 4 weeks) or placebo for up to one year.
The primary endpoint was DFS, while OS served as a hierarchically tested secondary endpoint. Exploratory endpoints included DMFS and PFS2. Safety and treatment-related adverse events (TRAEs) were also assessed throughout the study.
Patient Population
A total of 794 patients were randomized: 532 received nivolumab, and 262 received placebo. Patients had a median follow-up of 78.3 months (range 60.1–96.6), representing one of the longest follow-ups for adjuvant immunotherapy in this setting.
Efficacy Outcomes
Disease-Free Survival: Adjuvant nivolumab continued to demonstrate a sustained and statistically significant DFS benefit compared to placebo. Median DFS was 21.8 months (95% CI, 16.6–29.7) in the nivolumab arm versus 10.8 months (95% CI, 8.3–14.3) in the placebo arm, with a hazard ratio (HR) of 0.76 (95% CI, 0.63–0.91), reaffirming earlier findings.
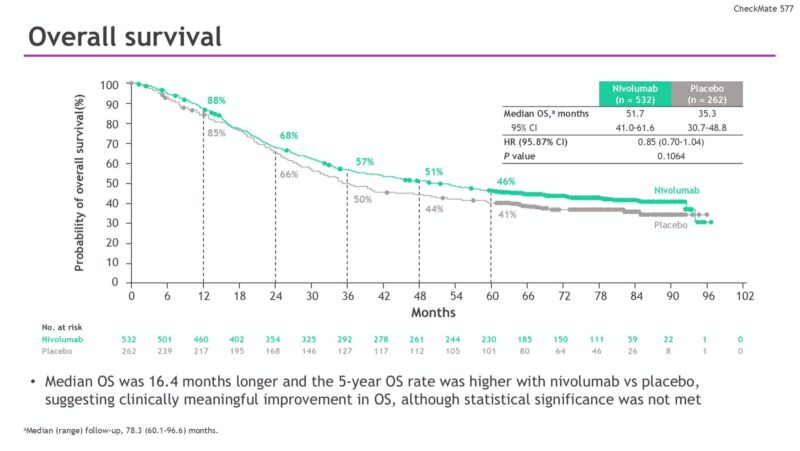
Overall Survival: Although the difference in median OS did not reach statistical significance at the final analysis, nivolumab showed a meaningful numerical improvement. Median OS was 51.7 months (95% CI, 41.0–61.6) for patients receiving nivolumab, compared with 35.3 months (95% CI, 30.7–48.8) in the placebo group (HR 0.85; 95.87% CI, 0.70–1.04; P = 0.1064). OS rates at 3 and 5 years were 57% and 46% with nivolumab versus 50% and 41% with placebo, respectively, suggesting a durable survival benefit.
Distant Metastasis-Free Survival: Nivolumab also conferred a significant improvement in DMFS, with median values of 27.3 months (95% CI, 21.4–36.0) versus 14.6 months (95% CI, 10.9–20.3) in the placebo arm (HR 0.75; 95% CI, 0.62–0.90). This indicates a substantial delay in distant recurrence among treated patients.
Progression-Free Survival 2 (PFS2): PFS2, measuring time to progression after subsequent systemic therapy, favored nivolumab with an HR of 0.81 (95% CI, 0.67–0.98), suggesting lasting benefits beyond initial adjuvant treatment.
Safety Profile
Treatment-related adverse events (TRAEs) were more common in the nivolumab arm but remained manageable. Any-grade TRAEs occurred in 71% of nivolumab-treated patients compared to 48% in placebo recipients. Grade 3–4 TRAEs were reported in 14% and 7%, respectively. Discontinuations due to TRAEs were slightly higher with nivolumab (9% any-grade, 5% grade 3–4) compared to placebo (3% and 3%). No new safety signals or unexpected toxicities emerged during the extended follow-up.
Clinical Significance
These findings from CheckMate 577 trial reinforce the role of adjuvant nivolumab as a new standard of care for patients with resected EC/GEJC with residual disease following neoadjuvant CRT. The durable DFS and improved DMFS suggest a meaningful impact on disease control, while the trend toward prolonged OS is encouraging.
With a favorable safety profile and manageable toxicity, nivolumab offers a significant therapeutic advance in this high-risk population, where recurrence rates remain historically high.
What They’re Saying: Reactions to CheckMate 577 Trial at ASCO 2025
Richard Dunne, MD, Leader, GI Oncologist from wilmotcancer center shared on X
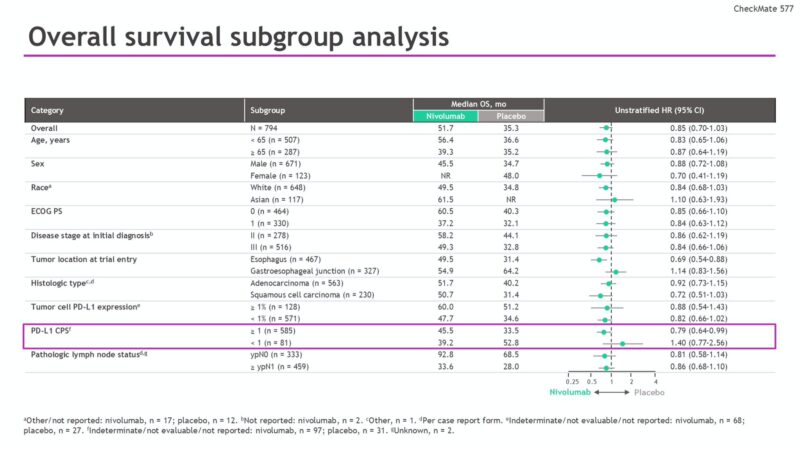
Checkmate 577 data final OS analysis. Key Subgroups reported: HR of 0.92 for OS in patients with esophageal adenocarcinoma with adjuvant nivolumab and tumors that are PDL1+ driving small benefit
Key Takeaway Messages from CheckMate 577 Trial
Adjuvant nivolumab significantly improved disease-free survival (DFS) compared to placebo in patients with resected stage II/III esophageal or gastroesophageal junction cancer with residual pathologic disease following neoadjuvant chemoradiotherapy. Median DFS was 21.8 months with nivolumab versus 10.8 months with placebo (HR 0.76, 95% CI 0.63–0.91).
Long-term data show a persistent DFS benefit with nivolumab after a median follow-up of over 78 months. The 3- and 5-year DFS rates remained higher for patients treated with nivolumab compared to placebo.
Overall survival (OS) showed a numerical improvement with nivolumab (median OS 51.7 vs 35.3 months for placebo), although the difference was not statistically significant at this final analysis (HR 0.85, 95% CI 0.70–1.04; P = 0.1064).
Distant metastasis-free survival (DMFS) and progression-free survival on subsequent therapy (PFS2) also favored nivolumab over placebo, further supporting its long-term benefit.
The safety profile of adjuvant nivolumab was manageable and consistent with previous reports, with no new safety signals observed over extended follow-up.
Fewer patients receiving nivolumab required subsequent therapy compared to placebo, and the need for subsequent immunotherapy was markedly lower in the nivolumab group.
You Can Watch More on OncoDaily Youtube TV
Written by Armen Gevorgyan, MD


