Presented by Dr. Lili Mao (Peking University Cancer Hospital, Beijing, China) at the American Society of Clinical Oncology (ASCO) Annual Meeting 2025, new findings from the phase II CAP 03-NEO trial highlight a promising neoadjuvant treatment strategy for patients with resectable stage II/III acral melanoma (AM), a rare and aggressive melanoma subtype more common in Asian and African populations.
What is the CAP 03-NEO Trial?
CAP 03-NEO (NCT05512481) is a prospective, two-stage clinical trial designed to evaluate the efficacy and safety of a triplet neoadjuvant regimen combining camrelizumab (a PD-1 inhibitor), apatinib (a VEGFR2 inhibitor), and temozolomide (an alkylating chemotherapy agent) in patients with resectable stage II or III acral melanoma. The trial builds upon prior success from the CAP 03 study, which showed this combination’s effectiveness in the advanced/metastatic setting, with a 64% objective response rate and median progression-free survival (PFS) of 18.4 months.
The CAP 03-NEO trial aims to determine whether this combination can induce major pathological responses when administered before surgery, aligning with emerging data from SWOG1801 and NADINA trials that support neoadjuvant over adjuvant immunotherapy in melanoma.
Trial Design and Patient Enrollment
This phase II study enrolled adult patients aged 18–75 years with resectable stage II/III acral melanoma across two treatment stages:
Stage I enrolled 30 patients who received:
- Camrelizumab 200 mg IV every 2 weeks (two 4-week cycles)
- Apatinib 250 mg orally once daily
- Temozolomide 200 mg/m² IV on Days 1–5 per cycle
Followed by surgical resection, and 15 cycles of adjuvant camrelizumab (every 3 weeks).
Stage II was triggered by the predefined response threshold in Stage 1, expanding enrollment to 30 additional patients with stage III disease receiving the same treatment regimen.
Baseline Characteristics
As of the December 2024 data cutoff, 30 patients were enrolled in Stage 1 of the CAP 03-NEO trial. The median age was 54 years (interquartile range: 41–61). Twenty-eight patients underwent surgery after neoadjuvant treatment. Two patients did not proceed to surgery—one due to personal choice, and the other because of newly diagnosed metastatic disease.
Key Results Presented at ASCO 2025
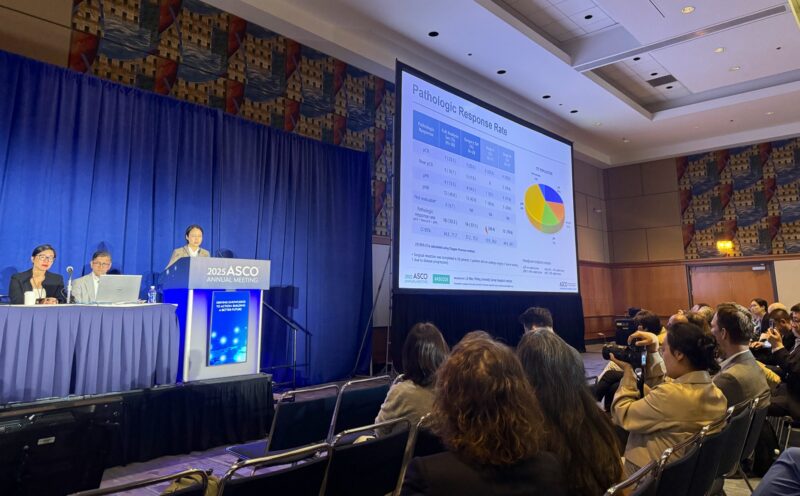
At the ASCO 2025 Annual Meeting, promising results from Stage 1 of the CAP 03-NEO trial were presented, highlighting the potential of neoadjuvant camrelizumab, apatinib, and temozolomide in resectable acral melanoma (AM). Among the 28 patients who underwent surgery, 57.1% (16 patients) demonstrated a pathological response to the triple-drug regimen.
A pathologic complete response (pCR) was achieved in 7 patients (25%), while 5 patients experienced near pCR and 4 showed a partial pathological response (pPR). Taken together, 42.9% (12 patients) achieved a major pathological response (MPR), defined as the sum of pCR and near-pCR.
When broken down by disease stage, outcomes were particularly promising in Stage III patients. Among the 17 patients with Stage III acral melanoma, 4 achieved a pCR, 5 reached near-pCR, and 3 experienced pPR, resulting in a pathological non-response rate (pNR) of just 29.4%. In contrast, of the 7 patients with Stage II disease, 3 achieved pCR and 1 had pPR, but the pNR rate was higher at 64%, indicating a more modest benefit in earlier-stage disease.
Survival outcomes further supported the efficacy of the regimen, with a 12-month event-free survival (EFS) rate of 74.1% (95% CI: 53.1–86.7%). At the time of data cutoff, the median EFS had not yet been reached, underscoring the durability of the treatment response.
These results highlight the potential of neoadjuvant camrelizumab, apatinib, and temozolomide in improving pathological and survival outcomes for patients with resectable acral melanoma—particularly in Stage III disease where treatment options remain limited and prognosis is often poor. Further investigation in larger cohorts is warranted to confirm these promising early findings.
Read Full Abstract on ASCO Official Website
Safety and Surgical Outcomes
The neoadjuvant regimen demonstrated a favorable safety profile, with no grade 4 or 5 adverse events reported. The most commonly observed side effects included elevated bilirubin levels (37%), decreased white blood cell counts (30%), and constipation (27%). Importantly, the addition of neoadjuvant therapy did not lead to an increase in surgical complications, reinforcing its feasibility and safety in the preoperative management of patients. These findings support the use of neoadjuvant treatment as a well-tolerated and effective approach in improving outcomes without compromising surgical safety.
What People Are Saying About CAP 03-NEO?
Dr. Patrick Hwu shared key highlights from the #ASCO25 melanoma rapid oral session on his X page, spotlighting results from the CAP 03-NEO trial presented by Dr. Lili Mao:
“More exciting updates live from this morning’s #melanoma rapid oral session at #ASCO25: Lili Mao, MD from @PKU1898 shares findings from the CAP 03-NEO trial on neoadjuvant camrelizumab + apatinib + temozolomide for resectable stage II/III acral #melanoma.
Key findings:
➡️ 25.0% pathological complete response (pCR)
➡️ 57.1% overall pathological response
➡️ 77.6% 12-month event-free survival; median EFS not yet reached
➡️ Manageable safety profile
➡️ No increase in surgical complications”

Key Takeaways from ASCO 2025
The CAP 03-NEO Stage 1 results demonstrate that neoadjuvant camrelizumab, apatinib, and temozolomide induce encouraging pathological responses in resectable acral melanoma—a disease with limited neoadjuvant data and high unmet need. The 42.9% MPR rate and manageable safety profile make a compelling case for this triplet regimen, particularly in stage III disease where pNR was lower.
With stage 2 enrollment ongoing, CAP 03-NEO may pave the way for a new standard of care for resectable AM, especially in Asian populations where acral melanoma is more prevalent.
More posts featuring ASCO25.