On September 9, 2025, Agenus Inc. (Nasdaq: AGEN) announced that its investigational immunotherapy combination, botensilimab (BOT) plus balstilimab (BAL), is now available to eligible patients with refractory microsatellite-stable (MSS) metastatic colorectal cancer (mCRC) through France’s compassionate access program (Accès Compassionnel, AAC).
The French National Agency for Medicines and Health Products Safety (ANSM) has approved hospital use of BOT/BAL for patients meeting specific criteria, including MSS status and no active liver metastases. Under this program, treatment is fully reimbursed by France’s Assurance Maladie, with hospitals compensated at the purchase price.
Garo Armen, PhD, Chairman & CEO of Agenus, said:
“This is a breakthrough for patients and their physicians. MSS colorectal cancer resists current immunotherapies, and treatment options after standard regimens are very limited,” said Garo Armen, PhD, Chairman & CEO of Agenus. “We are committed to supporting French centers to ensure a reliable supply and to collect high-quality real-world data.”

Photo of Garo Armen
How Does France’s Compassionate Access Work?
Once authorized, hospital use of BOT/BAL under AAC is covered 100% by Assurance Maladie. Hospitals are reimbursed at the invoiced purchase price, outside the standard Diagnosis-related Group (DRG), known in France as “en sus du GHS.” During AAC, the manufacturer either provides the therapy free of charge or charges a temporary indemnity, declared to the French Ministry. This mechanism ensures both patient access and compliance with national regulations.
Clinical Evidence Supports BOT/BAL Use
Patients with refractory MSS mCRC have few effective treatment options. Data from earlier studies show that the combination demonstrates durable anti-tumor activity, especially in patients without active liver metastases.
At ESMO GI 2025, updated results from the Phase 1b C-800-01 trial demonstrated that botensilimab plus balstilimab provides durable clinical benefit in patients with microsatellite-stable metastatic colorectal cancer (MSS mCRC) without active liver metastases.
The trial enrolled 123 heavily pretreated patients, with a median of three prior therapies. The combination achieved an objective response rate (ORR) of 20%, median overall survival (OS) of 20.9 months, and a clinical benefit rate at 24 weeks of 28%, with durable responses and a manageable safety profile.

Find more information on Botensilimab and Balstilimab and their durable survival in MSS colorectal cancer: ESMO GI 2025 update on OncoDaily.
What Trials Will Be Conducted in MSS Colorectal Cancer?
The Phase 3 BATTMAN trial (NCT07152821) will evaluate the combination of botensilimab (BOT) and balstilimab (BAL) versus Best Supportive Care (BSC) in patients with chemo-refractory, unresectable microsatellite-stable colorectal adenocarcinoma (MSS mCRC). Sponsored by the Canadian Cancer Trials Group, this pivotal study is designed to address a major unmet need for patients whose disease no longer responds to standard chemotherapy.
The trial is set to launch in November 2025 and plans to enroll 834 patients globally. Its primary endpoint is overall survival (OS), with secondary endpoints including progression-free survival (PFS), objective response rate (ORR), safety, and quality of life. The study will also explore tumor and blood biomarkers to help predict which patients derive the greatest benefit.
BATTMAN builds on encouraging early data from Phase 1b studies, where BOT/BAL demonstrated durable responses, long-term survival, and manageable safety in MSS mCRC patients—particularly those without active liver metastases. If successful, the BATTMAN trial could establish BOT/BAL as a novel immunotherapy option for heavily pretreated MSS colorectal cancer, expanding treatment options for this difficult-to-treat population.
Understanding Botensilimab and Balstilimab
Botensilimab (BOT) and Balstilimab (BAL) are investigational immunotherapies developed by Agenus Inc. Botensilimab (BOT) is a Fc-enhanced CTLA-4 antibody that stimulates both innate and adaptive immune responses. It works by:
- Activating T cells to recognize and attack tumor cells
- Reducing regulatory T cells (Tregs) within the tumor, which normally suppress immune activity
- Engaging myeloid cells to enhance anti-tumor immunity
- Inducing long-term immune memory for sustained responses
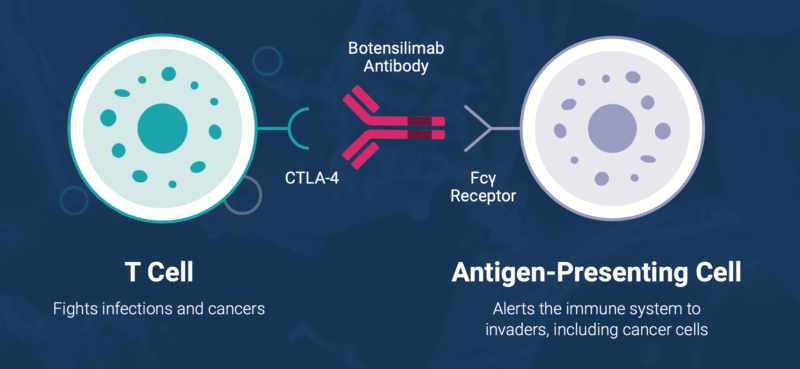
Balstilimab (BAL) is a PD-1 inhibitor that blocks the interaction between PD-1 on T cells and PD-L1/PD-L2 on tumor cells. This releases the “brakes” on T cells, allowing them to attack cancer more effectively. Together, BOT and BAL create a synergistic immune effect, targeting “cold” tumors that are usually resistant to standard immunotherapy. This combination has shown durable responses, long-term survival, and manageable safetyin MSS metastatic colorectal cancer and other solid tumors.
Other trials with BOT/BAL
On April 28, 2025, Agenus shared results from the Phase 2 NEOASIS study (NCT06279130), highlighting the efficacy of botensilimab and balstilimab (BOT/BAL) in the neoadjuvant setting across multiple solid tumors, including both mismatch repair–proficient (pMMR/MSS) and deficient (dMMR/MSI-H) cancers. These results mark the first reported neoadjuvant outcomes for BOT/BAL outside colorectal cancer.
The safety run-in enrolled 20 patients, divided into two cohorts: dMMR/MSI-H (n=10) and pMMR/MSS (n=10). Patients received botensilimab (25–50 mg) plus balstilimab (450 mg) on Day 1 and Day 22 before surgery.
Key Findings
- dMMR/MSI-H cohort: 90% achieved pathological response, with 70% complete responses and 80% major pathological responses.
- pMMR/MSS cohort: 80% had pathological responses, including 20% complete responses; triple-negative breast cancer patients achieved 63% major pathological responses.
- Safety: No dose-limiting toxicities or surgical delays were observed.
The findings were shared in an oral session at the AACR Annual Meeting in Chicago by Myriam Chalabi, MD, of the Netherlands Cancer Institute. This Phase 2 study enrolled patients with non-metastatic tumors, including colorectal cancer, triple-negative and ER-positive breast cancer, Merkel cell carcinoma, and sarcoma, divided into cohorts based on mismatch repair status.
UNICORN Phase II trial
The UNICORN Phase II trial, which results were published in the Journal of Clinical Oncology in 2025, highlights the potent neoadjuvant activity of botensilimab (BOT) alone or combined with balstilimab (BAL) in patients with resectable, locally advanced colon cancer.
This window-of-opportunity study enrolled 56 patients with mismatch repair–proficient (pMMR) or deficient (dMMR) tumors. Patients received a short course of botensilimab monotherapy or botensilimab plus balstilimab prior to scheduled surgery on day 35. The trial measured pathological response (pR), major response (pMR), and complete response (pCR), reflecting the proportion of viable tumor remaining.
Key Outcomes
- pMMR tumors: BOT alone showed minimal effect (pMR 0%, pR 43%), while BOT + BAL induced pCR 29%, pMR 36%, pR 71%.
- dMMR tumors: BOT monotherapy achieved pCR 29%, pMR 36%, pR 64%, but BOT + BAL reached pCR 93% and pMR 100%, marking the highest pCR reported in this population.
- Safety: Adverse events occurred in 50% of patients, mostly immune-related; serious treatment-related events were 5%. Surgery proceeded on schedule in nearly all patients.
These findings demonstrate that a single preoperative cycle of BOT/BAL can deliver remarkable pathological responses, supporting further exploration of non-operative management strategies in both pMMR and dMMR colon cancer.
Botensilimab plus Balstilimab in HCC
The Phase 1 BOT/BAL trial in hepatocellular carcinoma (HCC), with results presented at AACR 2025, highlights promising responses in heavily pretreated patients who had progressed on standard immunotherapies, including atezolizumab/bevacizumab.
The HCC cohort enrolled 19 patients (18 evaluable for efficacy) with metastatic, treatment-refractory disease. Patients received botensilimab (BOT) at 1–2 mg/kg plus balstilimab (BAL) 3 mg/kg, following progression on a median of two prior therapies, including anti-PD(L)-1 agents.
Key Results
- Overall response rate (ORR): 17%
- Stable disease (SD): 56%
- Disease control rate (DCR): 72%
- Median progression-free survival (PFS): 4.4 months
- Median overall survival (OS): 12.3 months
The treatment was well tolerated, with a safety profile consistent with prior BOT/BAL studies. These findings were presented by Dr. Anthony El-Khoueiry, MD, Associate Director for Clinical Research and Chief of Section of Developmental Therapeutics at the USC Norris Comprehensive Cancer Center, highlighting that BOT/BAL can provide durable responses even in advanced, immunotherapy-resistant HCC, supporting further evaluation in this high-need population.
Read about BOT/BAL Advancements, BATTMAN Trial, FDA Approval Challenges and Key Highlights from Agenus Stakeholder Briefing on OncoDaily.


