Dr. Benjamin Schlechter of Dana-Farber Cancer Institute presented updated results from the fully enrolled cohort of the ongoing Phase 1b C-800-01 trial (NCT03860272) with Botensilimab and Balstilimab during ESMO Gastrointestinal Cancers Congress 2025 in Barcelona.
The Phase 1b trial evaluated the regimen in microsatellite-stable metastatic colorectal cancer (MSS mCRC) patients without active liver metastases. The results showcased durable responses and long-term survival in a treatment-refractory population historically unresponsive to immunotherapy.
Background
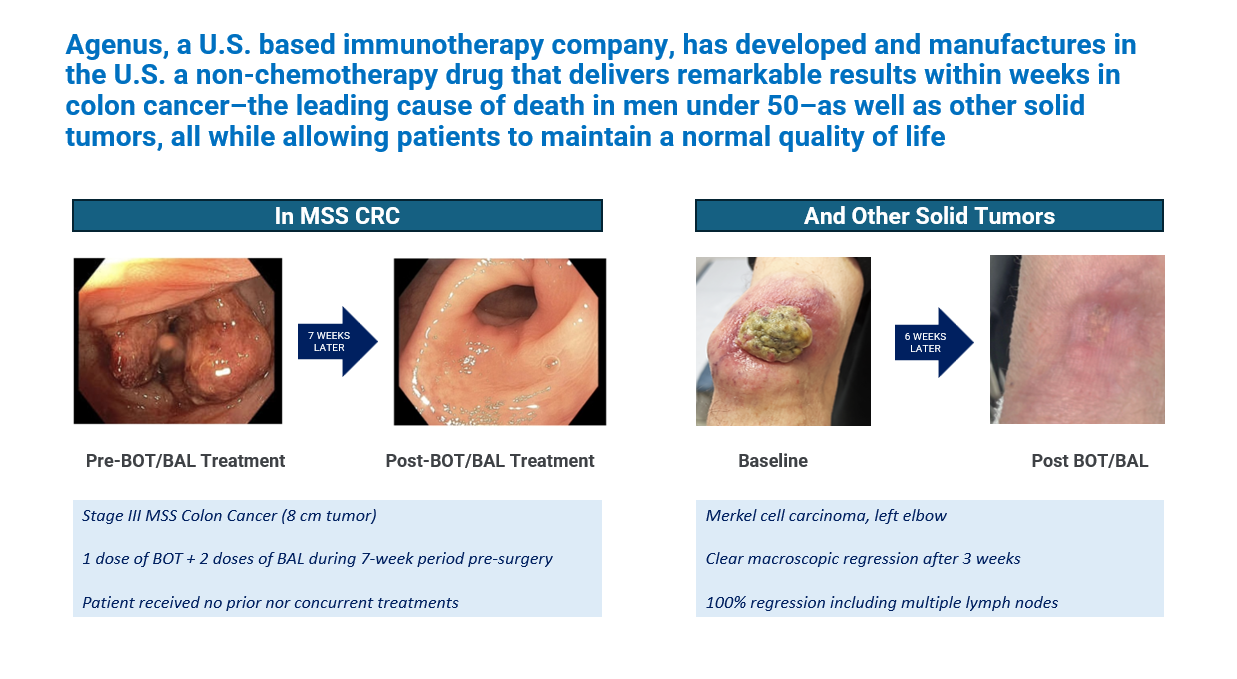
Microsatellite-stable metastatic colorectal cancer (MSS mCRC) has long posed a major treatment challenge due to its poor responsiveness to immune checkpoint inhibitors. For patients who progress after multiple lines of chemotherapy, therapeutic options remain scarce. Botensilimab (BOT), a multifunctional, Fc-enhanced CTLA-4 inhibitor, and balstilimab (BAL), an anti–PD-1 antibody, are designed to overcome these limitations through enhanced innate and adaptive immune activation. In prior reports, the BOT/BAL combination showed encouraging signs of efficacy in treatment-refractory MSS mCRC, particularly in patients without active liver metastases (NLM).
Study Design
The C-800-01 trial (NCT03860272) is an open-label, multicenter Phase 1b study evaluating botensilimab alone or in combination with balstilimab across multiple solid tumors, including a dedicated expansion cohort for microsatellite-stable metastatic colorectal cancer (MSS mCRC) patients without active liver metastases (NLM).
As of March 13, 2025, a total of 123 patients with advanced, treatment-refractory disease were enrolled into this cohort. Patients received botensilimab at either 1 mg/kg (n=62) or 2 mg/kg (n=61) every six weeks, combined with balstilimab 3 mg/kg every two weeks.
The primary objectives included safety, objective response rate (ORR), and progression-free survival (PFS), with overall survival (OS) and duration of response (DOR) as key secondary endpoints. The trial also allowed for exploratory analyses of immune-related adverse events (imAEs) and treatment-free survival, with follow-up extending beyond 18 months. No maximum tolerated dose was reached, and safety was evaluated across both dosing arms.
Key Results
In the fully enrolled expanded cohort of 123 patients with microsatellite-stable metastatic colorectal cancer (MSS mCRC) and no active liver metastases (NLM), the combination of botensilimab (BOT) and balstilimab (BAL) demonstrated clinically meaningful efficacy, with durable responses, a survival plateau, and a manageable safety profile—all in a population largely resistant to prior therapies.
The cohort was heavily pretreated:
- Median age: 56 years (range 25–82)
- Median prior lines of therapy: 3 (range 1–10)
- 67% had RAS mutations
- 16% had received prior immunotherapy;
- 30% had prior regorafenib or trifluridine/tipiracil ± bevacizumab or fruquintinib
- 58% had ≥1 metastatic site
- 16% had previously treated liver metastases
The objective response rate (ORR) was 20% (24/123; 95% CI: 13–28), including:
- 3 complete responses (CR)
- 21 partial responses (PR)
ORR by dose level:
- BOT 1 mg/kg: 19%
- BOT 2 mg/kg: 20%
The clinical benefit rate at 24 weeks (CR, PR, or stable disease) was 28% (34/123; 95% CI: 20–36). The median duration of response (DoR) was 16.6 months (95% CI: 5.7–not reached), reflecting sustained benefit among responders.
Key survival outcomes:
- Median progression-free survival (PFS): 4.0 months (95% CI: 2.8–4.1)
- Median overall survival (OS): 20.9 months (95% CI: 16.2–26.6)
- 18-month OS rate: 57% (95% CI: 48–66)
Importantly, 20% of patients (24/123) were alive and off treatment at data cut-off (median follow-up: 18.0 months, range: 0.7–53.3 months), suggesting treatment-free survival and potential for durable immune-mediated disease control.
The safety profile was manageable and aligned with expected immune-related effects. Immune-mediated adverse events (imAEs) occurred in 58% of patients, including 29% Grade 3 and 1% Grade 4 events. The most frequent imAE was diarrhea/colitis (41%, with 15% Grade 3 and 1% Grade 4). At the selected BOT 1 mg/kg dose, the imAE rate dropped to 45% (Grade 3: 24%), with diarrhea/colitis reduced to 29% (Grade 3: 11%).
There were no treatment-related deaths and no new safety signals reported.
Agenus Confirms Positive FDA Interaction and Launch of Phase 3 BATTMAN Trial
On July 1, 2025, Agenus held an End-of-Phase 2 (EoP2) meeting with the U.S. Food and Drug Administration (FDA) to discuss the regulatory path for botensilimab plus balstilimab (BOT/BAL) in microsatellite-stable metastatic colorectal cancer (MSS mCRC). The FDA acknowledged that current clinical data “appears to support” the contribution of balstilimab to the combination’s clinical activity, thereby allowing Agenus to proceed with a Phase 3 registration trial without a BOT monotherapy control arm—a significant regulatory concession that materially de-risks the trial design.
The agency and company also aligned on the core structure of the global BATTMAN Phase 3 trial (CCTG CO.33), which will evaluate BOT/BAL in MSS mCRC with overall survival as the primary endpoint. The trial is set to launch in Q4 2025.

Read More About Bal/Bot program, Agenus, FDA Approval on OncoDaily
Looking Ahead: 2H2025 Clinical and Regulatory Milestones
-
BATTMAN Trial Launch (Q4 2025)
-
Earlier-Line CRC Expansion studies underway
-
Global Clinical Network Growth, including strategic partnerships
-
New Data Presentations in Q4 2025
-
Regulatory Engagement to secure expedited approval
About the BOT/BAL Program
The C-800-01 Phase 1 study (NCT03860272) enrolled over 1,200 patients across multiple solid tumor types. Botensilimab (BOT), a multifunctional, Fc-enhanced CTLA-4 inhibitor, and balstilimab (BAL), an anti–PD-1 antibody, have demonstrated encouraging clinical activity in nine different cancers, including those typically unresponsive to standard immune checkpoint therapies.
What drugs are Botensilimab (BOT) and Balstilimab (BAL)?
BOT is a novel Fc-enhanced CTLA-4 inhibitor designed to activate both innate and adaptive immune responses, especially in “cold” tumors—traditionally resistant to standard immunotherapies. It primes and activates T cells, reduces intratumoral regulatory T cells, stimulates myeloid cells, and promotes durable memory responses.
BAL, Agenus’s PD-1 inhibitor, blocks the PD-1 receptor interaction with its ligands PD-L1 and PD-L2, enhancing T-cell activation and immune-mediated tumor destruction. Combined, BOT/BAL has demonstrated potent and durable anti-tumor activity across multiple challenging cancers, notably colorectal and hepatocellular carcinoma (HCC).
You Can Read More ESMO Gastrintestinal Cancer Congress 2025 Highlights on Oncolodaily.


