At the recent Gateway for Cancer Research Cures Gala, one story captured the hearts of everyone in the room — not because it was about illness, but because it was about hope, science, and the power of compassion.
Behind the bright lights and moving speeches stood a quiet revolution in cancer care: the BOT/BAL immunotherapy program at Duke University, led by Dr. Nicholas DeVito and supported by Gateway for Cancer Research. This innovative trial is rewriting what’s possible for patients with microsatellite-stable (MSS) colorectal cancer, one of the most treatment-resistant cancer types.
From Chemistry to Compassion
Botensilimab (BOT) and Balstilimab (BAL) are next-generation investigational immunotherapies developed by Agenus Inc., designed to awaken the immune system against hard-to-treat cancers.
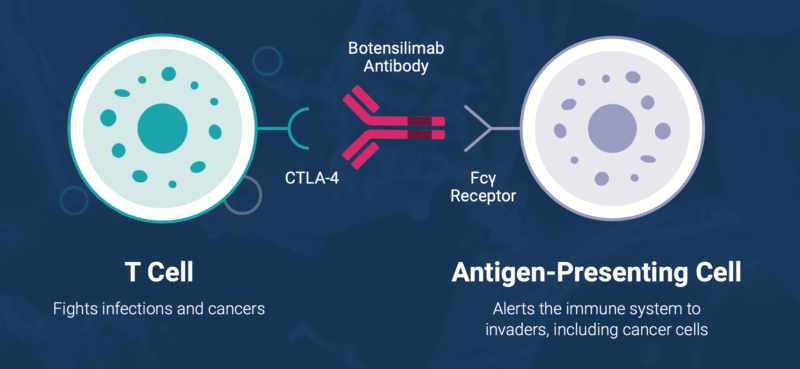
Botensilimab is an Fc-enhanced CTLA-4 antibody that stimulates both the innate and adaptive arms of immunity. It helps the body recognize and sustain its fight against cancer by:
- Activating T cells to identify and destroy tumor cells
- Reducing suppressive regulatory T cells (Tregs) within the tumor microenvironment
- Engaging myeloid cells to strengthen anti-tumor activity
- Building long-term immune memory for durable responses
Balstilimab complements this action as a PD-1 inhibitor, blocking the interaction between PD-1 on T cells and PD-L1/PD-L2 on tumor cells. By releasing these “immune brakes,” it restores T-cell function and allows the immune system to attack more effectively.
Together, BOT and BAL create a synergistic immune response capable of transforming “cold” tumors—those traditionally resistant to immunotherapy—into responsive ones. This combination has already demonstrated durable responses, prolonged survival, and manageable safety in patients with microsatellite-stable metastatic colorectal cancer and other solid tumors.
The Role of Gateway for Cancer Research
None of this would have been possible without Gateway for Cancer Research, a nonprofit organization that accelerates early-phase, practice-changing clinical trials. Gateway’s mission is simple but profound — to shape a world in which a cancer diagnosis is no longer feared. By funding bold ideas and empowering patients, Gateway turns philanthropy into tangible breakthroughs.
As Interim CEO Michael Jessup shared during the Gala,
“Every dollar is a promise — a promise to patients waiting for answers and to families holding on to hope.”
At its 34th Annual Cures Gala, supporters raised millions to advance early-stage studies like BOT/BAL — proving that compassion can power discovery.
A Global Wave of Progress
In September 2025, France became the first country to grant reimbursed compassionate access for BOT/BAL to patients with refractory MSS metastatic colorectal cancer (mCRC).
Approved by the French National Agency for Medicines (ANSM), this program allows hospitals to prescribe BOT/BAL under Accès Compassionnel (AAC) — fully reimbursed by Assurance Maladie.
Eligible patients (MSS status, no active liver metastases) can now receive this therapy outside clinical trials, a milestone that underscores France’s leadership in facilitating early access to breakthrough treatments.
“This is a breakthrough for patients and their physicians,”
said Garo Armen, PhD, CEO of Agenus.
“We’re committed to ensuring reliable supply and real-world data collection.”
The French decision builds on data from the Phase 1b C-800-01 trial, presented at ESMO GI 2025, where BOT/BAL achieved:
- Objective Response Rate (ORR): 20%
- Median Overall Survival (OS): 20.9 months
- 24-week Clinical Benefit Rate: 28%
all in heavily pretreated MSS mCRC patients — with a manageable safety profile.

Read about France Grants Reimbursed Compassionate Access for BOT/BAL in Refractory MSS Colorectal Cancer on OncoDaily.
Durable Survival Across Solid Tumors
Beyond colorectal cancer, BOT/BAL is emerging as a pan-tumor platform.
At ESMO 2025, results from the Phase 1b basket trial (NCT03860272) involving 411 patients with refractory solid tumors — including MSS CRC, NSCLC, sarcoma, ovarian, and hepatocellular carcinoma — showed:
- Objective Response Rate: 17%
- Median Duration of Response: 15 months
- Median Overall Survival: 17 months
- 2-year OS rate: 39%
Patients who developed immune-related side effects actually lived longer — a hallmark of active immune engagement.

Read more about Dual CTLA 4 and PD 1 Blockade With Botensilimab and Balstilimab Demonstrates Durable Survival in Refractory Solid Tumors on OncoDaily.
A Story of Courage and Family
Among the Duke University trial participants were two men whose stories moved everyone who watched the Gateway Cures Gala video — Greg Friedman and Spencer Laird. Their journeys captured what science alone cannot express: the quiet strength of families facing the impossible and the hope that emerges when compassion meets innovation.
Greg had already seen cancer take too much from his life — his mother, his first wife, and several relatives — before hearing his own diagnosis. Spencer, a husband and father of a five-year-old daughter, discovered his colorectal cancer during a routine check, only to learn later that it had spread to his lungs. Surgery was no longer possible.
“They told us chemo and radiation were the only options,” Spencer recalled. “But we thought there had to be something better.”
That “something better” arrived through the Gateway-supported BOT/BAL immunotherapy trial at Duke, led by Dr. Nicholas DeVito. Designed to awaken the immune system against microsatellite-stable colorectal cancer — a form long resistant to treatment — the therapy offered a new kind of hope.
“It’s basically training your immune system to do what it’s supposed to do anyway,” Greg explained. “After the first scans, some of the smaller tumors were gone — just gone.”
Dr. DeVito described what he and his team witnessed as a first for microsatellite-stable colorectal cancer — patients maintaining durable control without chemotherapy. The early results, he said, are more than clinical data; they represent proof that immune resistance can be broken.
Today, both Greg and Spencer continue their immunotherapy, living life fully and gratefully — symbols of what happens when research, philanthropy, and faith align to turn possibility into reality.
The Broader Meaning
Immunotherapy has already transformed outcomes in melanoma and lung cancer, but MSS colorectal cancer has long remained resistant to immune-based approaches. The early BOT/BAL experience at Duke now offers hope that this barrier can finally be broken.
Beyond the science, this story is a reminder of what happens when researchers, donors, and patients unite around one belief — that cures begin with courage.
Gateway’s mission is not only to fund research, but to fuel possibility. For every patient who dares to hope, every scientist who keeps searching, and every supporter who gives from the heart — progress is no longer theoretical. It’s real, and it’s happening now.


