Clinical Trial ID: NCT03860272
Presenter: Michael Gordon, MD (Scottsdale, USA)
Funding and Sponsor: Agenus Inc.
Co-authors: Apostolia Maria Tsimberidou, Benjamin L. Schlechter, Breelyn Wilky, Marwan Fakih, Andrea Bullock, Ghassan K. Abou-Alfa, Jonathan W. Goldman, Robin L. Jones, Justin Stebbing, Dhan Chand, Wei Wu, Benny Johnson, Joseph Grossman, Steven J. O’Day, Anthony B. El-Khoueiry.
Dual immune checkpoint blockade targeting CTLA-4 and PD-1 has historically produced deep and durable responses in highly immunogenic cancers such as melanoma. However, most solid tumors remain poorly immunogenic and resistant to standard CTLA-4 ± PD-1 therapy.
Botensilimab (BOT), a novel Fc-enhanced CTLA-4 inhibitor, was designed to broaden immune activation through multiple mechanisms, while balstilimab (BAL), a PD-1 inhibitor, complements this effect. Together, they aim to extend the durable benefits of checkpoint inhibition—such as long-term survival plateaus and prolonged stable disease—to patients with heavily pretreated and refractory solid tumors.

Methods
This phase Ib, multicenter basket trial (NCT03860272) enrolled 411 patients with advanced, treatment-refractory solid tumors across multiple types, including microsatellite-stable metastatic colorectal cancer, sarcomas, non-small cell lung cancer, ovarian cancer, hepatocellular carcinoma, and others.
Patients received:
- Botensilimab at either 1 mg/kg or 2 mg/kg every 6 weeks, and
- Balstilimab at 3 mg/kg every 2 weeks.
Most participants were heavily pretreated, with a median of 3 prior lines of therapy (range 0–17), and 33% were immune-oncology (I-O) refractory.
The data cutoff was December 5, 2024, with a median follow-up of 10 months (range 0.5–53.3).
Results
Among 343 evaluable patients, the combination demonstrated meaningful and durable antitumor activity:
- Objective Response Rate (ORR): 17% (95% CI 13–21), consistent across both BOT doses
- Median Duration of Response: 15 months (range 10 – not reached)
- Clinical Benefit Rate (CR + PR + SD ≥ 24 weeks): 26%
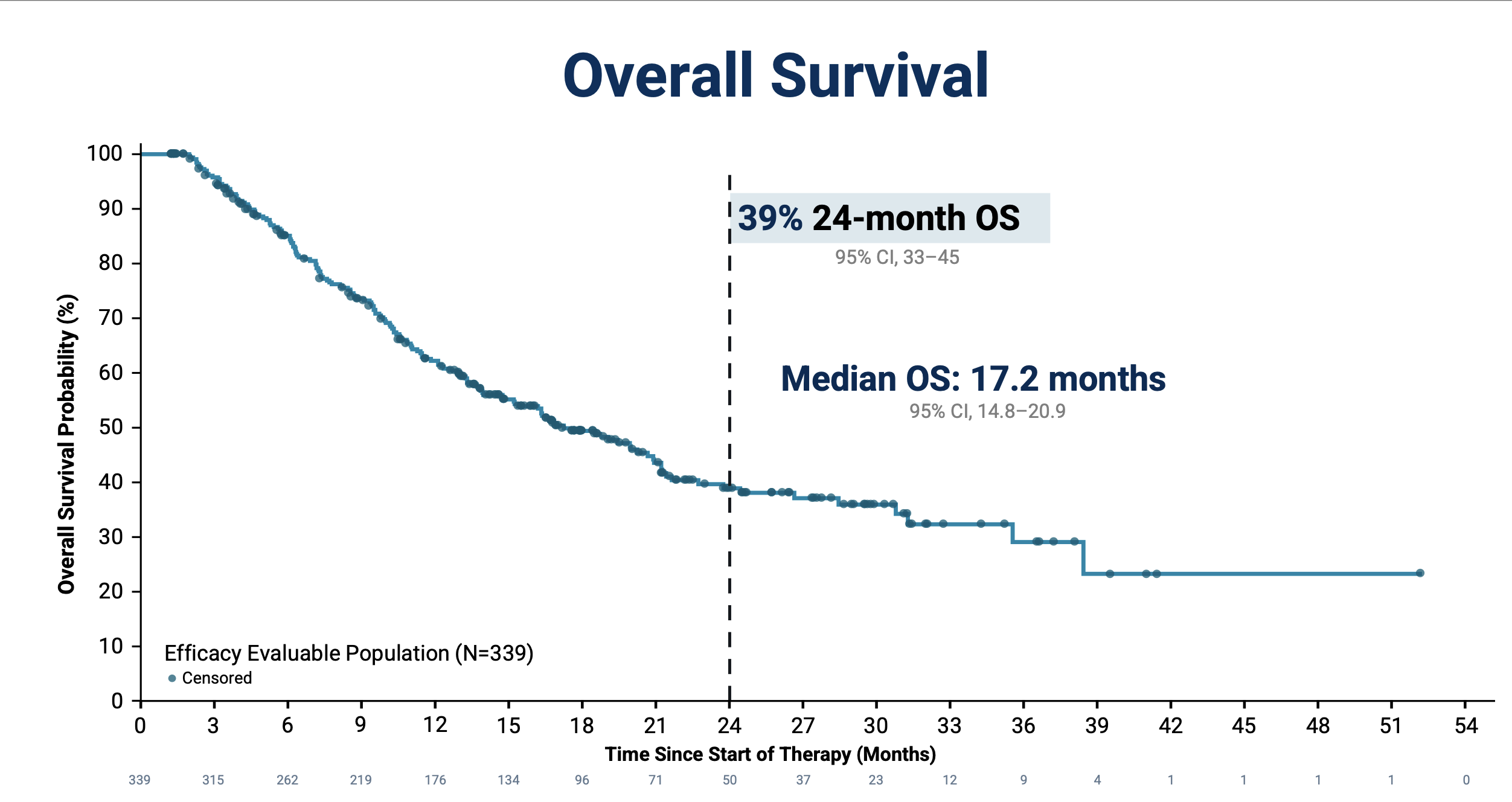
- Median Overall Survival (mOS): 17 months (95% CI 14–21)
- 24-month OS rate: 39% (95% CI 32–45), showing the emergence of long-term survival plateaus
Activity by subgroup:
I-O-naïve vs I-O-refractory:
- ORR 19% vs 12%
- mOS 21 vs 12 months
- 24-mo OS 42% vs 30%
Non-active vs active liver metastases:
- ORR 19% vs 13%
- mOS 20 vs 12 months
- 24-mo OS 42% vs 29%
Safety profile:
- Immune-mediated adverse events (imAEs): 47% (any grade), 17% grade 3, 1% grade 4
- Diarrhea/colitis: 33% (any grade), 10% grade 3
- No treatment-related deaths reported
- Interestingly, patients who developed imAEs or discontinued therapy due to toxicity had better survival
outcomes:
- With imAEs vs none: mOS 24 vs 14 months; 24-mo OS 49% vs 31%
- With discontinuation vs none: mOS 24 vs 16 months; 24-mo OS 49% vs 37%
Conclusions
The combination of botensilimab + balstilimab produced deep and durable responses and notable 2-year survival plateaus across a variety of refractory solid tumors, including those traditionally unresponsive to immunotherapy.
The observation that immune-mediated toxicity correlated with improved overall survival reinforces the relationship between immune activation and long-term benefit.
These late-stage, pan-tumor results complement emerging neoadjuvant studies and highlight botensilimab + balstilimabas a promising next-generation immunotherapy platform capable of extending survival in resistant solid tumors.
You Can Watch More on OncoDaily Youtube TV


