Artificial intelligence (AI) in oncology refers to the use of advanced computer algorithms and machine learning techniques to analyze vast and complex cancer-related data. AI systems are designed to mimic aspects of human intelligence—such as learning from experience, recognizing patterns, and making predictions. In the field of oncology, AI is transforming how cancer is detected, diagnosed, treated, and monitored. By processing large volumes of medical images, pathology slides, genomic sequences, and electronic health records, AI can identify hidden patterns, support clinical decision-making, and personalize treatment strategies. The integration of AI into cancer care is driving a new era of precision medicine, enabling earlier detection, more accurate diagnosis, and improved outcomes for patients facing a cancer diagnosis.
How Is AI Used in Cancer Diagnosis?
Artificial intelligence (AI) is revolutionizing the field of cancer diagnosis by dramatically improving the speed, accuracy, and consistency of detecting malignancies. Today, AI is integrated into multiple steps of the diagnostic process, supporting clinicians in both routine screening and complex, ambiguous cases.
AI in Medical Imaging
Artificial intelligence has become a powerful asset in radiology, particularly through deep learning systems capable of interpreting medical images such as X-rays, CT scans, MRIs, and mammograms. These algorithms are trained on massive datasets and can detect subtle abnormalities—sometimes even before they’re visible to the human eye. In breast cancer screening, for instance, AI has demonstrated accuracy on par with or exceeding expert radiologists, leading to earlier and more reliable detection. In lung cancer, AI can analyze low-dose CT scans to spot small pulmonary nodules, helping to catch malignancies in their most treatable stages.
Similarly, in brain and liver imaging, AI tools help define tumor boundaries more precisely, which is crucial for diagnosis, treatment planning, and surgical approaches. A landmark study published in Nature showed that Google Health’s AI outperformed human experts in mammogram interpretation, significantly reducing false negatives and positives.
Digital Pathology
AI is revolutionizing pathology by analyzing high-resolution digital images of biopsy samples. After tissue slides are digitized, machine learning models can examine the microscopic architecture of cells and tissues to distinguish benign from malignant changes and even classify cancer subtypes. This process not only speeds up diagnosis but also reduces variability between pathologists. AI-powered digital pathology is making it possible to process and interpret complex cases more consistently and accurately. Companies like PathAI and Paige are pioneering this technology, and their tools are being increasingly adopted in major hospitals and academic centers.
Genomic and Molecular Diagnostics
In the era of precision medicine, AI is invaluable for sifting through enormous genomic datasets to identify mutations, gene expression patterns, and other molecular features linked to cancer. AI platforms can rapidly interpret next-generation sequencing (NGS) data to pinpoint actionable mutations that help confirm diagnosis and guide targeted therapy. These systems can also integrate molecular findings with clinical and imaging data to provide a more comprehensive understanding of a patient’s disease. As a result, oncologists are better equipped to select personalized treatment options and enroll patients in appropriate clinical trials.
Symptom and Risk Assessment Tools
AI doesn’t just analyze images and tissue samples—it can also mine electronic health records using natural language processing to flag patients who might be at increased risk of cancer. By evaluating a combination of symptoms, family history, and previous laboratory results, AI systems can suggest which individuals should be prioritized for further screening or diagnostic workup. These tools help clinicians catch cancer in earlier, more treatable stages and optimize resource allocation.
Decision Support for Clinicians
Integrated clinical decision support systems powered by AI are increasingly being used in oncology. These platforms pull together a patient’s imaging, pathology, laboratory, and genomic information to suggest potential diagnoses or highlight areas needing additional investigation. They provide oncologists with evidence-based recommendations, improving diagnostic accuracy and supporting multidisciplinary decision-making.
The Role of AI in Cancer Imaging
Artificial intelligence is revolutionizing cancer imaging by bringing unprecedented accuracy and speed to the detection, characterization, and monitoring of tumors. Traditional imaging modalities—such as X-ray, CT, MRI, PET, and ultrasound—are indispensable in oncology, but interpreting these images can be challenging due to subtle findings, inter-reader variability, and ever-increasing data volumes. AI addresses these challenges by leveraging machine learning and deep learning techniques to analyze images in ways that complement and enhance radiologist expertise.
One of the earliest and most impactful applications of AI in cancer imaging has been in screening for breast, lung, and prostate cancers. Deep learning models trained on large datasets can identify malignancies on mammograms, CT scans, and MRIs with sensitivity and specificity matching or exceeding that of expert radiologists. For example, studies have shown that AI can reduce false-negative rates in breast cancer screening and detect early lung nodules on low-dose CT scans, contributing to earlier diagnoses and better patient outcomes (Yala et al., 2021, NPJ Breast Cancer; Ardila et al., 2019, Nature Medicine).
AI also plays a pivotal role in tumor segmentation, measuring tumor size and volume, and monitoring changes over time. These tasks are essential for assessing treatment response and planning surgery or radiation. Automated segmentation powered by AI ensures greater reproducibility and efficiency compared to manual methods (Hosny et al., 2018, Nature Reviews Cancer).
Radiomics, a field combining imaging and computational analysis, uses AI to extract hundreds of quantitative features from standard scans. These features provide insights into tumor heterogeneity, microenvironment, and biology, which can inform prognosis and therapy selection. In practice, AI-driven radiomics models have been used to predict which patients will respond to immunotherapy or targeted treatments, enabling a more personalized approach to care (Aerts et al., 2014, Nature Communications).
Furthermore, AI supports the triage of imaging studies by flagging suspicious findings for urgent review, reducing time to diagnosis for critical cases. In pathology, AI assists with the analysis of digital slides, supporting the detection of micrometastases and rare cancer subtypes that might be overlooked by the human eye (Campanella et al., 2019, Nature Medicine). By integrating information from imaging, genomics, and electronic health records, AI is enabling the development of comprehensive, data-driven tools that support diagnostic accuracy, reduce human error, and optimize care pathways across the cancer continuum.
AI and Cancer Screening Programs
Artificial intelligence is reshaping cancer screening programs by enhancing detection accuracy, reducing human error, and making large-scale population screening more efficient and cost-effective. The integration of AI into screening programs for breast, lung, colorectal, cervical, and prostate cancers is already yielding substantial benefits, both in clinical trials and real-world practice.
Breast Cancer Screening
AI-powered algorithms have demonstrated performance comparable to expert radiologists in interpreting mammograms. Large studies show that deep learning models can reduce false negatives and false positives, identify subtle lesions missed by humans, and help standardize interpretations across institutions. For instance, a multi-center study led by McKinney et al. (2020, Nature) found that Google’s deep learning system outperformed radiologists in both the UK and US datasets, reducing false positives by 5.7% and 1.2%, respectively, and false negatives by 9.4% and 2.7%.
How AI Enhances Mammography for Early Breast Cancer Detection
One of the most transformative applications of artificial intelligence in oncology is its role in enhancing cancer screening programs, particularly mammography for early breast cancer detection. The image above illustrates how AI-driven tools assist radiologists in interpreting mammograms with greater accuracy and efficiency.
In this example, AI software automatically highlights suspicious regions on the mammogram with a red box, directing the radiologist’s attention to potential areas of concern. The image on the left shows an early lesion that may not be easily visible to the human eye. The image on the right demonstrates a confirmed malignant mass in the same region, now clearly detected with the aid of AI.
This technology leverages deep learning algorithms trained on thousands of annotated mammogram images to identify subtle patterns associated with malignancies. The key benefits include:
AI-supported mammography has been shown in studies to improve breast cancer detection rates and reduce false negatives, particularly in women with dense breast tissue. According to recent trials published in The Lancet Oncology and Radiology, AI-assisted mammography can achieve sensitivity rates that match or exceed double reading by human radiologists.
By integrating AI into routine mammography screening workflows, healthcare systems aim to catch breast cancer at earlier stages, improving survival outcomes while alleviating the diagnostic burden on radiologists.
This example highlights how AI is already making a tangible impact in real-world cancer screening, offering patients the potential for earlier diagnosis and treatment.
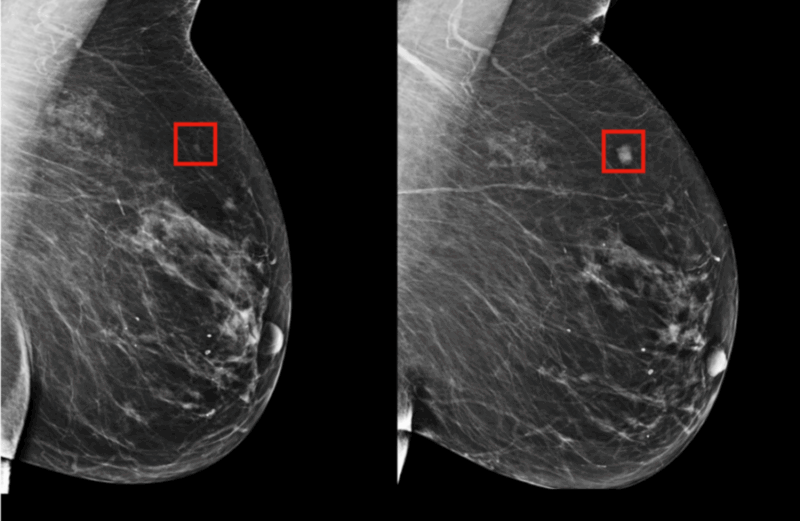
Photo From Massachusetts Institute of Technology
Lung Cancer Screening
Low-dose computed tomography (LDCT) is the standard for lung cancer screening in high-risk populations. AI tools can identify lung nodules and assess malignancy risk more accurately and rapidly than traditional reading. Ardila et al. (2019, Nature Medicine) showed that a deep learning system could match or exceed the diagnostic accuracy of expert radiologists for early-stage lung cancer, helping prioritize follow-up and reduce missed diagnoses.
Colorectal and Other Cancers
AI is also improving the sensitivity and specificity of colorectal cancer screening by automating polyp detection during colonoscopy (Urban et al., 2018, Nature Biomedical Engineering). AI-assisted colonoscopy systems have been associated with higher adenoma detection rates, which are linked to reduced colorectal cancer mortality. Similarly, AI is being piloted in cervical cancer screening through automated interpretation of Pap smears and in prostate cancer through analysis of MRI and biopsy images (Litjens et al., 2017, Nature Reviews Urology).
Impact on Access and Equit
AI can expand access to high-quality cancer screening in underserved areas by enabling non-specialist clinicians to perform and interpret screening studies with accuracy similar to experts. AI-powered mobile devices and cloud-based tools are being used in low- and middle-income countries to support cervical and breast cancer screening, helping to close gaps in global cancer care (Mitani et al., 2023, The Lancet Oncology).
AI and Precision Oncology: Personalizing Cancer Treatment
The rise of precision oncology is reshaping cancer care by tailoring treatments to the unique genetic and molecular features of each patient’s tumor. Artificial intelligence is at the heart of this transformation, enabling clinicians and researchers to interpret complex, multidimensional datasets and translate them into actionable insights for personalized therapy.
AI-driven algorithms analyze genomic sequencing results, gene expression profiles, and proteomic data at a speed and scale unattainable by humans. By integrating these molecular findings with clinical records and imaging, AI can identify mutations or biomarkers that predict sensitivity or resistance to specific therapies (Kourou et al., 2015, BioMed Research International). For instance, machine learning platforms are used to match patients with targeted therapies or immunotherapies, selecting treatments most likely to yield a response based on their molecular profile.
In a landmark study, IBM Watson for Oncology demonstrated the ability to recommend treatment regimens for breast cancer that were concordant with expert tumor boards in over 90% of cases, highlighting AI’s potential to assist clinical decision-making (Somashekhar et al., 2018, The Oncologist).
Furthermore, AI models are being developed to predict how a patient will respond to chemotherapy, radiotherapy, or emerging immunotherapies by analyzing clinical, genomic, and radiomic data (Esteva et al., 2019, Nature Medicine). These predictive models enable oncologists to avoid ineffective treatments and minimize unnecessary toxicity, increasing both the efficacy and safety of cancer therapy. As an example, deep learning tools can stratify patients with non-small cell lung cancer (NSCLC) based on their likelihood of benefiting from immunotherapy, supporting more personalized and cost-effective treatment plans (Ardila et al., 2019, Nature Medicine).

In clinical trials, AI is accelerating the identification of new drug targets and biomarkers by analyzing real-world evidence and high-throughput screening data (Topol, 2019, Nature Medicine). AI platforms are also improving patient selection for clinical studies, ensuring that trials enroll participants most likely to benefit from investigational treatments.
Overall, AI-powered precision oncology is ushering in a new era where cancer therapy is increasingly customized to the biological fingerprint of each tumor. This shift not only improves patient outcomes but also optimizes healthcare resources, as demonstrated in recent studies and real-world clinical practice (Yu et al., 2022, CA: A Cancer Journal for Clinicians).
Can AI Predict Cancer Outcomes?
Artificial intelligence (AI) has shown significant promise in predicting cancer outcomes, including prognosis, risk of recurrence, survival rates, and response to therapy. By analyzing large and complex datasets that integrate clinical, imaging, genomic, and pathological information, AI models can identify subtle patterns and prognostic factors that may not be evident to clinicians alone (Kourou et al., 2015, BioMed Research International).
Machine learning algorithms are being used to develop predictive models for various cancers. For example, deep learning systems can analyze histopathology images and radiology scans to forecast the likelihood of recurrence or metastasis in breast, lung, prostate, and colorectal cancers (Mobadersany et al., 2018, NPJ Digital Medicine). AI can also assess electronic health records, laboratory values, and molecular biomarkers to create risk stratification tools, supporting oncologists in making individualized follow-up and treatment decisions (Esteva et al., 2019, Nature Medicine).
A prominent example is the use of AI in breast cancer. Researchers have developed AI tools that predict five-year recurrence risk by integrating patient demographics, tumor characteristics, and imaging features. These models often match or exceed the performance of traditional clinical risk calculators, such as Adjuvant! Online and PREDICT (Yala et al., 2021, NPJ Breast Cancer). Similarly, in lung cancer, AI-driven radiomics models can forecast patient survival based on CT scan features and genetic markers, providing valuable information for treatment planning (Aerts et al., 2014, Nature Communications).
Importantly, AI is also being used to predict response to specific cancer therapies, such as immunotherapy, by analyzing patterns in tumor DNA, RNA expression, and the tumor microenvironment (Jiang et al., 2018, Nature Medicine). This allows for a more personalized approach to treatment and helps avoid ineffective therapies.
While AI models are not infallible and require validation in diverse patient populations, they are already supporting clinical decision-making in major cancer centers and are expected to become a standard component of oncology care in the near future (Yu et al., 2022, CA: A Cancer Journal for Clinicians).
AI in Cancer Clinical Trials: Streamlining Recruitment and Analysis
Artificial intelligence is playing an increasingly important role in the design, recruitment, and analysis of cancer clinical trials. One of the major challenges in oncology research is finding eligible patients efficiently, interpreting complex data from diverse sources, and accelerating the path from discovery to clinical implementation. AI-driven solutions are addressing these hurdles by automating processes and providing insights that would be impossible to achieve manually.
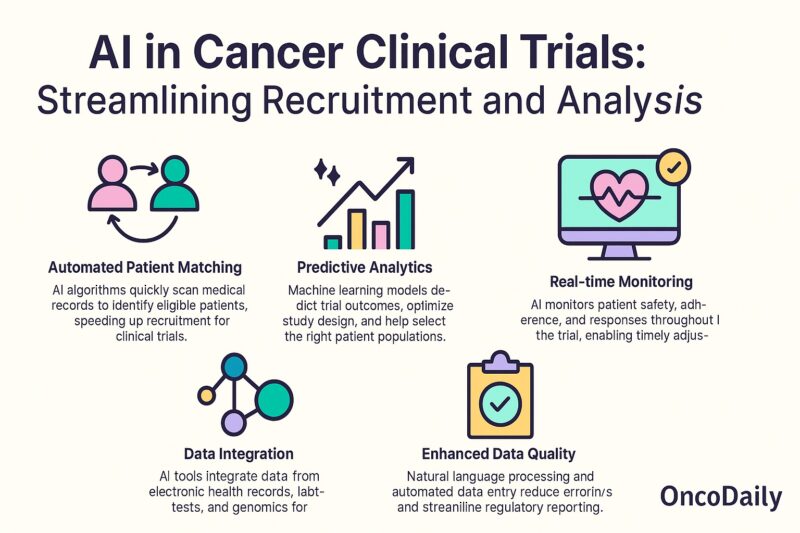
AI-powered algorithms can rapidly scan and interpret electronic health records (EHRs), genomic databases, and unstructured clinical notes to match patients with relevant trials based on disease subtype, biomarker profiles, treatment history, and eligibility criteria. This significantly reduces the time and resources needed for recruitment, a process that is traditionally slow and often leads to trial delays or failures (Miho et al., 2022, Journal of Clinical Oncology). For instance, natural language processing (NLP) tools extract information from clinical documents to automate patient prescreening, enabling real-time identification of potential trial candidates (Duma et al., 2022, JAMA Oncology).
Beyond recruitment, AI is revolutionizing trial design and data analysis. Machine learning models can predict trial outcomes, identify subgroups of patients most likely to benefit, and optimize adaptive trial designs by continuously analyzing accruing data (Liu et al., 2021, The Lancet Digital Health). In addition, AI supports the analysis of real-world evidence by integrating data from EHRs, imaging, and genomics, allowing researchers to monitor treatment effectiveness and safety outside of controlled trial environments (Cheng et al., 2021, Nature Medicine).
These advancements improve trial efficiency, lower costs, and enhance the scientific validity of findings. AI is also helping to address disparities in trial access by identifying and enrolling more diverse patient populations (Patel et al., 2021, Journal of the American Medical Informatics Association).
Real-World Applications: Hospitals and Startups Leading the Way
The integration of artificial intelligence into oncology practice is rapidly transitioning from research to real-world applications, driven by both leading academic hospitals and innovative startups. AI technologies are now being adopted in cancer centers around the world to enhance diagnostic accuracy, streamline workflow, and personalize patient care.
Major Hospitals and Cancer Centers
Top institutions such as the Mayo Clinic, MD Anderson Cancer Center, and Memorial Sloan Kettering Cancer Center have established dedicated AI research teams and digital pathology platforms. For example, the Mayo Clinic has deployed deep learning algorithms to assist in the interpretation of radiology and pathology images, which has improved detection rates and reduced diagnostic errors (Yala et al., 2021, NPJ Breast Cancer).
MD Anderson has integrated IBM Watson for Oncology into its workflow, demonstrating that AI-based clinical decision support can align with expert tumor board recommendations in most breast and colorectal cancer cases (Somashekhar et al., 2018, The Oncologist). Memorial Sloan Kettering’s collaboration with Paige has enabled the adoption of AI-powered pathology tools that support the identification of rare cancer subtypes and improve reproducibility (Campanella et al., 2019, Nature Medicine).
Startup Innovation and Commercial Solutions
Startups are accelerating the pace of AI adoption through scalable, commercial solutions. PathAI, for instance, has developed machine learning pathology platforms now deployed in clinical laboratories and pharmaceutical research (Pantanowitz et al., 2020, Journal of Pathology Informatics). Paige is another pioneer, having received FDA approval for its AI-based prostate cancer detection tool, which assists pathologists in identifying malignant tissue with greater sensitivity and efficiency (Campanella et al., 2019, Nature Medicine). In radiology, Aidoc and Zebra Medical Vision provide AI-based triage and detection systems for lung nodules, brain metastases, and breast cancer, with proven improvements in workflow and early detection rates (Lakhani & Sundaram, 2017, Radiology).
Benefits of AI in Oncology
The integration of artificial intelligence into oncology is rapidly transforming cancer care by improving accuracy, efficiency, and personalization throughout the patient journey. One of the most profound benefits is enhanced diagnostic precision. AI algorithms trained on millions of radiology and pathology images can recognize subtle patterns and abnormalities that may escape even expert eyes. For example, in breast and lung cancer screening, AI systems have been shown to reduce both false negatives and false positives, leading to earlier detection and fewer unnecessary interventions (Yala et al., 2021, NPJ Breast Cancer; Ardila et al., 2019, Nature Medicine).
Beyond diagnostics, AI accelerates and improves the analysis of complex molecular and genomic data, supporting the identification of actionable mutations and the selection of targeted therapies. In precision oncology, this capacity enables clinicians to customize treatment plans to each patient’s tumor biology, increasing the likelihood of response and reducing exposure to ineffective treatments (Kourou et al., 2015, BioMed Research International).
Workflow efficiency is another key advantage. AI-powered tools automate time-consuming tasks, such as triaging imaging studies, flagging urgent cases, and pre-screening patients for clinical trial eligibility. This allows clinicians to spend more time on direct patient care and decision-making. In pathology, AI expedites slide analysis, reduces turnaround times, and helps standardize diagnostic criteria across institutions (Campanella et al., 2019, Nature Medicine).
Patient outcomes are also improving as a result of AI adoption. AI-supported decision tools synthesize vast amounts of patient data to generate evidence-based recommendations, supporting multidisciplinary tumor boards and individual oncologists in delivering optimal care (Somashekhar et al., 2018, The Oncologist). Early evidence suggests that AI can help reduce disparities in cancer care by increasing access to high-quality diagnostics in resource-limited settings (Yu et al., 2022, CA: A Cancer Journal for Clinicians).
Finally, AI contributes to drug discovery and clinical trial efficiency. Machine learning models can predict which drug candidates are most likely to succeed, identify optimal patient cohorts for new therapies, and continuously analyze trial data to refine study designs. This streamlines the development of new cancer treatments, potentially bringing life-saving therapies to patients faster.
Challenges and Limitations of AI in Cancer Care
While artificial intelligence (AI) is transforming oncology, its integration into cancer care is not without significant challenges and limitations. Understanding these issues is essential for clinicians, researchers, and policymakers working to ensure that AI delivers on its promise to improve outcomes for all patients.
Data Quality and Bias
AI algorithms are only as good as the data on which they are trained. Many AI models are developed using data from single institutions, specific populations, or controlled research environments, which can limit their generalizability. If the data are incomplete, poorly annotated, or unrepresentative, the resulting models may perpetuate existing healthcare disparities or perform less well in diverse clinical settings (Parikh et al., 2019, JAMA Oncology). For example, a deep learning system trained predominantly on images from White populations may be less accurate for patients of other ethnicities.
Regulatory and Validation Hurdles
Unlike traditional diagnostic tools, AI systems continuously evolve as they process new data. This presents unique regulatory challenges. Demonstrating robust clinical validation, reproducibility, and ongoing safety is required for regulatory approval. However, a lack of standardized frameworks and clear pathways for AI devices can slow clinical adoption (Topol, 2019, Nature Medicine).
Transparency and Interpretability
The “black box” nature of many deep learning models can undermine clinician and patient trust. When AI tools provide a diagnosis or recommendation, clinicians may not understand how the output was derived. This lack of interpretability makes it challenging to explain decisions, manage legal risks, or identify the source of errors (He et al., 2019, Nature Medicine).
Ongoing Need for Human Oversight
AI is a tool to augment, not replace, oncologists. Human expertise is still critical for contextualizing AI recommendations, managing unexpected results, and providing compassionate care. Studies consistently find that the best results occur when AI and clinicians work collaboratively, not in isolation (Jiang et al., 2017, JAMA).
Real-World Examples: Addressing AI Challenges in Oncology
Improving Data Quality and Reducing Bias: The Memorial Sloan Kettering Cancer Center collaborated with companies like Paige to aggregate pathology images from global sources, building diverse datasets that enhance the robustness and fairness of AI models (Campanella et al., 2019, Nature Medicine). The Cancer Imaging Archive (TCIA) is a public repository providing open access to a wide range of annotated imaging data, which supports the development and external validation of AI tools for all populations (Clark et al., 2013, Cancer Research).
Regulatory and Validation Efforts
The U.S. FDA launched the Digital Health Innovation Action Plan and has approved several AI-based imaging and pathology devices (FDA, 2022). This initiative encourages continuous real-world monitoring, performance benchmarking, and transparent reporting. International coalitions such as the Medical Device Innovation Consortium (MDIC) are working on standards for AI validation and post-market surveillance.
Clinical Workflow Integration
The Mayo Clinic piloted AI tools in radiology with close collaboration between clinicians and IT teams, offering extensive training and integrating AI outputs directly into radiologist workstations to minimize workflow disruptions (Yala et al., 2021, NPJ Breast Cancer). Epic and Cerner, leading EHR vendors, have built AI “marketplaces” for seamless deployment of clinical algorithms within EHR systems, increasing usability and adoption.
Privacy and Ethics
Hospitals like Massachusetts General Hospital use “federated learning,” a privacy-preserving approach where AI models are trained on data at multiple hospitals without moving data across institutional boundaries, reducing privacy risks (Rieke et al., 2020, Nature Medicine).
The Future of AI in Cancer Care
Artificial intelligence is poised to play an even greater role in transforming every facet of cancer care—from prevention and early detection to treatment selection, monitoring, and survivorship. The next wave of AI innovation will likely bring more precise, equitable, and efficient care to patients around the globe.

Toward Truly Personalized Oncology
Future AI systems will harness multi-omics data—integrating genomics, transcriptomics, proteomics, radiomics, and electronic health records—to create comprehensive “digital twins” of each patient. These digital models will simulate disease progression and treatment response, allowing clinicians to select the most effective, least toxic therapies for each individual (Topol, 2019, Nature Medicine; Bibault et al., 2021, The Lancet Oncology).
Real-Time Decision Support and Adaptive Therapy
Emerging AI platforms will provide real-time clinical decision support, continuously updating recommendations based on the latest evidence, patient data, and outcomes from similar patients. Adaptive clinical trials, powered by AI, will accelerate the development of new cancer drugs by dynamically matching patients to novel therapies based on evolving tumor characteristics (Esteva et al., 2021, Nature).
Early Detection and Risk Prediction
AI’s role in cancer screening will continue to expand. Next-generation algorithms will not only interpret imaging and pathology but also integrate wearable device data, blood biomarkers, and environmental risk factors to predict cancer risk and detect recurrence earlier than ever before (Yala et al., 2021, NPJ Breast Cancer; Aerts, 2023, Nature Reviews Cancer).
Empowering Patients and Survivors
AI-driven mobile apps and wearable devices will give patients new tools for symptom tracking, medication adherence, mental health support, and personalized survivorship care, enabling a more proactive, patient-centered approach to long-term health.
Most Popular AI Bots and Tools in Cancer Care
As artificial intelligence continues to transform oncology, an increasing number of AI-powered bots, platforms, and tools are being integrated into clinical practice and cancer research. Below are 10 of the most widely used and promising AI tools currently shaping cancer care:
Paige.AI: leverages deep learning to analyze pathology slides with high accuracy, particularly in breast, prostate, and colorectal cancers. Its FDA-approved Paige Prostate Detect is used to assist pathologists in detecting cancer.

PathAI: provides advanced pathology AI solutions that improve diagnostic accuracy and workflow efficiency. It helps pathologists interpret complex biopsy samples, supporting more accurate treatment decisions.
Tempus: offers a precision medicine platform that integrates genomic sequencing, clinical data, and AI to help oncologists personalize treatment plans and match patients to suitable clinical trials.
IBM Watson for Oncology: One of the first AI systems applied in oncology, IBM Watson assists clinicians by analyzing medical literature and patient data to suggest evidence-based treatment options. It is widely used in several hospitals globally.
Arterys: provides cloud-based AI radiology tools, including solutions for cancer imaging. It enables faster and more accurate interpretation of CT and MRI scans for lung and breast cancer.

Freenome: Freenome uses AI to analyze cell-free DNA in blood samples to enable early cancer detection. It is particularly focused on non-invasive screening for colorectal cancer and other early-stage malignancies.
PathAI BioPharma: beyond clinical pathology, PathAI also partners with pharmaceutical companies to optimize AI-driven biomarker discovery and companion diagnostics in oncology drug development.
Aidoc: offers AI tools for radiology that help triage and prioritize oncological imaging findings in real time. It is being used in emergency and routine cancer imaging workflows.

SOPHiA GENETICS: provides an AI-based analytics platform that supports multi-modal data interpretation for precision oncology, combining genomics, radiomics, and clinical data.
OncoHost: OncoHost leverages proteomic analysis and AI to predict patient response to immunotherapy and guide personalized treatment strategies in various cancers, helping reduce ineffective treatments.
These innovative AI bots and platforms are streamlining cancer screening, enhancing diagnostic precision, supporting personalized treatment planning, and accelerating drug development. As AI adoption in oncology grows, these tools—and many new ones on the horizon—will play a pivotal role in shaping the future of cancer care.
You Can Watch More on OncoDaily Youtube TV
Written by Armen Gevorgyan, MD
FAQ
How is AI used in cancer diagnosis?
AI enhances cancer diagnosis by analyzing medical images, pathology slides, and genomic data, helping detect tumors and genetic mutations with high accuracy.
Can AI detect cancer earlier than traditional methods?
Yes, AI can detect subtle patterns in imaging and biomarkers, enabling earlier cancer detection, which is critical for improving survival rates.
What is precision oncology and how does AI support it?
Precision oncology uses AI to analyze individual tumor profiles and patient data, allowing oncologists to tailor treatment plans for maximum effectiveness.
Is AI used in cancer screening programs?
AI is increasingly integrated into cancer screening programs, such as mammography for breast cancer, CT scans for lung cancer, and colonoscopy image analysis.
Can AI predict cancer outcomes?
AI models can analyze large datasets to predict cancer progression, treatment response, and patient outcomes, supporting better clinical decision-making.
How does AI streamline cancer clinical trials?
AI accelerates trial recruitment by identifying eligible patients, enhances study design through predictive analytics, and improves data quality and real-time monitoring.
What are the benefits of using AI in oncology?
AI offers faster diagnosis, more accurate risk stratification, personalized treatment recommendations, and improved efficiency in clinical workflows.
What are the limitations and challenges of AI in cancer care?
Challenges include data privacy concerns, potential bias in AI models, limited availability of high-quality training data, and regulatory hurdles.
Are AI tools replacing oncologists?
No. AI tools are designed to assist, not replace, oncologists. They provide decision support and automate repetitive tasks, allowing doctors to focus on patient care.
What are some of the top AI tools used in oncology?
Popular tools include Google Health’s mammography AI, PathAI, Tempus, IBM Watson for Oncology, Aidoc, Paige AI, and Zebra Medical Vision.


