Angiogenesis remains a central driver of disease progression in metastatic colorectal cancer, particularly in the chemorefractory setting where therapeutic options are limited and largely molecular-agnostic. While several antiangiogenic strategies are approved in later lines, predictive biomarkers of benefit are lacking. Cabozantinib, an oral multi-kinase inhibitor targeting VEGFR2, MET, and AXL, has demonstrated activity across multiple tumor types and may be particularly relevant in mCRC characterized by mesenchymal features such as epithelial–mesenchymal transition (EMT) and immune evasion.
In March 2026, ESMO Gastrointestinal Oncology published the final clinical and translational results of the phase II ABACO trial, evaluating cabozantinib in patients with metastatic colorectal cancer who had progressed on all standard therapies. The study provides both efficacy data and exploratory molecular correlates of response in this heavily pretreated population.
Title: Clinical and translational results from the phase II ABACO trial evaluating the activity of cabozantinib in pretreated patients with metastatic colorectal cancer
Authors: V. De Falco, P.P. Vitiello, D. Ciardiello, G. Grasso, E. Mariella, G. Martini, S. Napolitano, C. Cardone, G. Arrichiello, E. Varriale, M. Di Bisceglie, T. Latiano, E. Maiello, A. Reginelli, M.C. Brunese, S. Cappabianca, S. Del Tufo, A. Orlando, A. Nicastro, F. Caraglia, L. Esposito, D. Renato, A. Avallone, M. Orditura, N. Fazio, G. Curigliano, M.G. Zampino, A. Bardelli, F. Ciardiello, T. Troiani, E. Martinelli
Read more about Cabozantinib on OncoDaily.
Methods
ABACO was a single-center, open-label, single-arm phase II trial conducted in Italy. Eligible patients were adults with histologically confirmed mCRC, ECOG performance status 0–1, measurable disease per RECIST v1.1, and documented progression after fluoropyrimidines, oxaliplatin, irinotecan, anti-VEGF therapy, and anti-EGFR agents when appropriate.
Patients received cabozantinib 60 mg orally once daily, with dose reductions permitted for toxicity. The primary endpoint was the progression-free survival (PFS) rate at 16 weeks. Secondary endpoints included median PFS, overall survival (OS), objective response rate (ORR), disease control rate, and safety. Exploratory analyses incorporated comprehensive genomic profiling and RNA sequencing to identify potential molecular determinants of response.
The trial followed a Simon two-stage design, with ≥30% PFS at 16 weeks predefined as clinically meaningful activity.
Results
Between October 2019 and January 2023, 33 patients were assessable for efficacy. The median age was 58 years, 57.6% were male, and all patients had microsatellite-stable disease. The majority of patients (63.6%) had received more than two prior lines of systemic therapy (median, three lines), and liver metastases were present in 78.8% of cases.
Efficacy
The study met its primary endpoint.
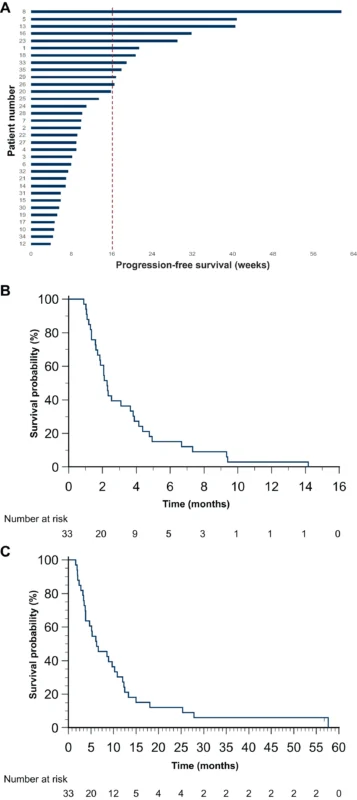
- 33% of patients (11/33) were progression-free at 16 weeks
- Median PFS: 2.27 months (95% CI 1.71–3.65)
- Median OS: 6.25 months (95% CI 3.81–10.26)
- ORR: 3% (one partial response)
- Disease control rate: 45.5%
No significant correlation between RAS/BRAF status, sidedness, or clinical features and PFS was observed.
Safety
Cabozantinib was generally well tolerated. The most frequent adverse events included fatigue, transaminase elevation, hand–foot syndrome, hypothyroidism, and diarrhea. Grade ≥3 adverse events occurred in 17% of patients. Dose reductions were required in approximately 40%, and treatment discontinuation due to toxicity occurred in three patients. No unexpected safety signals emerged.
Translational analyses
Comprehensive genomic profiling was available for 30 patients. While no single genomic alteration clearly enriched for response, two molecular features were significantly associated with improved PFS:
- Absence of TP53 mutations
- Tumor mutational burden ≥4 mutations/Mb
RNA sequencing was successfully performed in 18 patients, showing that CMS4 was the most prevalent consensus molecular subtype, consistent with an aggressive, mesenchymal phenotype. Importantly, tumors from patients achieving prolonged PFS showed a trend toward higher transcriptional activation of EMT and angiogenesis pathways, suggesting potential biological alignment between cabozantinib’s mechanism of action and tumor dependency.
These findings were exploratory but hypothesis-generating.
Read about Cure Rates in Metastatic Cancer on OncoDaily.
Conclusion
Within the limitations of a single-arm phase II design, the ABACO trial demonstrates that cabozantinib is active and safe in heavily pretreated metastatic colorectal cancer. Approximately one-third of patients achieved durable disease control beyond 16 weeks, with efficacy observed across molecular backgrounds.
Translational analyses suggest that baseline tumor biology—particularly EMT and angiogenesis activation—may identify tumors with a higher likelihood of clinical benefit from cabozantinib. Absence of TP53 mutations and TMB ≥4 mutations/Mb were associated with improved PFS in univariate and multivariate analyses. These results support further prospective evaluation of cabozantinib, alone or in combination, and highlight the potential value of transcriptional profiling in refining treatment strategies for chemorefractory mCRC.
The full article is available in ESMO Gastrointestinal Oncology.




