The second week of November brought important updates across GI oncology, with new data emerging from hepatobiliary, pancreatic, colorectal, esophageal, and liver cancer research. From Milan to Houston, Cambridge to Ankara, Hong Kong to California, teams shared evidence reshaping diagnosis, treatment strategies, and translational understanding.
Across leading journals — Liver International, Clinical Cancer Research, Nature Reviews Clinical Oncology, ESMO Open, and Nature Communications — researchers reported advances in real-world immunotherapy, biomimetic nanoparticle platforms, tumor heterogeneity, MASLD-driven hepatocarcinogenesis, and precision oncology.
Below is a curated selection of the week’s most impactful posts from leaders in GI oncology and translational research.
Andrea Casadei Gardini, MD – Associate Professor, Università Vita-Salute San Raffaele (Italy)
“New Publication!
Thrilled to share our latest work published in Liver International:
‘Durvalumab in Advanced Biliary Tract Cancer: Real-World Data From a Large Cohort of Patients Across Multiple International Centers’
This is the largest global real-world study on the combination of cisplatin, gemcitabine, and durvalumab in advanced biliary tract cancer, including 1,358 patients treated across 55 centers in 12 countries (Europe, U.S., and Asia).Key findings:
• Median OS: 15.6 months, PFS: 7.6 months
• ORR: 35.6%, DCR: 82.7%
• Prognostic factors: albumin > 3.5 g/dL, ECOG 0, NLR < 3, normal CEA, prior surgeryThese results confirm the efficacy and safety of the chemo-immunotherapy regimen observed in the phase III TOPAZ-1 trial, further supporting its use in routine clinical practice.
A heartfelt thanks to all 55 participating institutions and to our outstanding international collaborators from Europe, Asia, and the U.S. for making this global effort possible.”
Miguel Pereira-Silva, PhD – Research Scientist, Nanomedicine & Biomimetic Nanoparticles, MD Anderson Cancer Center (USA)
“Check out our Review Article on Biomimetic NPs for #pancreaticcancer treatment!
This review examines recent progress in biomimetic nanosystems designed to improve therapeutic outcomes in pancreatic cancer.
Key approaches include cell-membrane–coated nanoparticles, vesicle-based carriers, and hybrid bio-inspired platforms.The article outlines their:
mechanisms of action,
reported advantages in targeting and immune evasion
compares their performance across preclinical models
Major translational challenges—such as manufacturing consistency, scalability, and regulatory validation—are also discussed! You can find the link below:”
Alberto Puccini, MD – Medical Oncologist, Humanitas Cancer Center, Milan (Italy)
“I’m thrilled to share our new paper, ‘Impact of RAS/BRAF V600E mutations on the tumor immune microenvironment in mismatch repair deficient / microsatellite instability colorectal cancers’, now published in Clinical Cancer Research.
It has been a long journey, but it has truly paid off!It has been an honor to co-lead this project with my friend and colleague Mohamed Salem!
A heartfelt thank you to all our co-authors, true key opinion leaders in the field, for their collaboration, commitment, and tremendously valuable contributions that made this work possible.”
Chris Jones, MD – Clinical Lecturer in Clinical Oncology, University of Cambridge (UK)
“Spatial and temporal heterogeneity are pervasive causes of poor outcomes in oesophageal adenocarcinoma; challenging disease prevention, early diagnosis and treatment.
In our latest publication, in Nature Reviews Clinical Oncology, we explore the biology and therapeutic implications of this diversity from premalignant through to invasive disease.
We discuss:
How heterogeneity develops and progresses in Barrett oesophagus, and what this means for risk stratification.
The value of spatially distributed and repeat sampling for refining risk prediction models.
How intra- and inter- tumoural heterogeneity impede the development of effective treatments.
Emerging strategies to counteract these challenges, including targeting drivers of intratumoural diversity, developing evolution-directed and combination therapies, and improving tumour monitoring to identify targets for targeted therapies.”
Cihad Tatar, MD, FACS, FEBS-C, FTBS – General Surgeon
“I am pleased to announce that our Delphi study entitled ‘International expert Delphi consensus on management of early and locally advanced rectal cancer’ has been officially published in the International Journal of Colorectal Disease.
I extend my sincere gratitude to all the co-authors, the TSCRS Rectal Cancer Study Group experts, and our international colleagues for their invaluable contributions.”

April Choi, MD – Gastrointestinal Medical Oncologist & HS Assistant Clinical Professor, University of California Irvine (USA)
“Did you know that California has one of the highest burdens of hepatocellular carcinoma (HCC) incidence in the United States? This underscores the importance of having accessible and high-quality clinical trials for our patients.
At UCI Health, we are proud to offer a broad range of HCC trials—from UCI 23-75 (ROUTE90), which uses Eye90 microspheres for locoregional disease, to UCI 16-94, combining transarterial tirapazamine embolization (TATE) with immunotherapy (both led by Dr. Nadine Abi-Jaoudeh!).
I am excited to contribute to these efforts as the site principal investigator for ETCTN 10703: A Phase I/II Trial of Sapanisertib in Combination with Cabozantinib in β-catenin–mutated Hepatocellular Carcinoma, which was activated this week.”
Terence Lee, PhD – Professor, The Hong Kong Polytechnic University (Hong Kong SAR)
“We are excited to share our paper published in Nature Communications, which highlights the crucial role of wild-type KRAS in enabling hepatocellular carcinoma (HCC) cells to evade detection and destruction by T cells. Although KRAS mutations in HCC are rare, limiting their exploration as a therapeutic target, our findings present a novel strategy to enhance the effectiveness of current immunotherapies by targeting wild-type KRAS.”
Vivek Subbiah, MD – Chief, Early-Phase Drug Development, Sarah Cannon Research Institute (USA)
“Hot off the press!
Thrilled to share my editorial ‘All roads lead to ROME: convergence of evidence in precision oncology’ — in ESMO – European Society for Medical Oncology journal ESMO Open on the landmark ROME trial published in Nature Portfolio journal NATURE MEDICINE.Huge congratulations to the brilliant ROME investigators!”

Arndt Vogel, MD – Clinician-Scientist, ESMO Ambassador, focussed on Liver Cancer & Precision Oncology, Toronto General Hospital/Princess Margaret Cancer Center
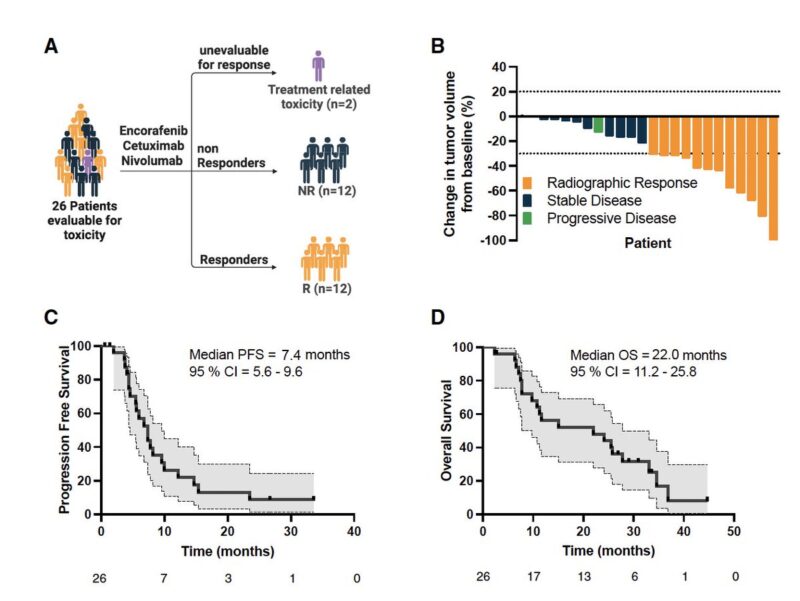
“Phase 1/2 trial of encorafenib, cetuximab, and nivolumab in MSS BRAF V600E mCRC
ORR 50%, mPFS 7.4 mo
Subgroup of mCRC with baseline MAPK activation & immune activation signatures may benefit from the triplet”
İmdat Eroğlu, MD, PhD – Medical Oncologist, Gazi University; PhD in Medical Biology & Genetics; ASCO Trainee & Early Career Advisory Group Member; Steering Committee Member, Young Cancer Professionals (European Cancer Organisation) – Türkiye
“In our recent bicentric study, we evaluated the efficacy and safety of neoadjuvant FLOT in Turkish and German patients.
Our findings revealed that clinical node status, rather than ethnicity, was the main determinant of major pathological response. Moreover, intensified neoadjuvant treatment (>4 cycles) did not improve pathological outcomes and was even associated with worse survival.I am deeply grateful to our co-authors from University Hospital of Bonn, especially Dr. Christian Möhring and Prof. Maria A. Gonzalez-Carmona, for this valuable collaboration.”

You can also read about 10 Must-Read Posts in GI Oncology from the First week of November on OncoDaily.



