In cancer medicine, the next wave of breakthroughs often appears long before regulatory approval. These investigational therapies generate momentum at major scientific meetings, shift treatment expectations, and signal where oncology is heading. The goal is not hype, but anticipation—because patients, clinicians, and researchers want to understand which emerging medicines are already demonstrating strong efficacy, meaningful survival gains, or first-in-class mechanisms. What they share is simple: their early data suggest they are capable of reshaping treatment standards in the near future.
Last year, many of the therapies highlighted by the OncoDaily Research & Intelligence team went on to receive FDA approval in 2025, while others continue to progress toward regulatory decisions. This year, we again reviewed the latest data, and the OncoDaily Research & Intelligence Editorial Team has selected the 10 most promising cancer drugs not yet approved—agents showing meaningful survival gains, impressive response rates, and first-in-class mechanisms in some of the most difficult cancers to treat.

Most Promising Cancer Drugs 2024
10 most promising cancer drugs not yet approved in 2025
1. Vepdegestrant (ARV-471)
2. Darovasertib
3. Relacorilant
4. Daraxonrasib
5. Zanzalintinib
6. GSK5764227 (HS-20093)
7. Anbenitamab (KN026)
8. AgenT-797 (iNKT) .
9. Pasritamig (JNJ-78278343)
10. Iza-Bren (EGFRxHER3 ADC)
These programs are shaping the next wave of innovation and offering a clear preview of what may redefine oncology care in the near future.

1. Vepdegestrant (ARV-471): Rewriting Endocrine Therapy for ER-Positive Breast Cancer
Indication: ER+/HER2- Advanced or Metastatic Breast Cancer (specifically ESR1-mutated).
Mechanism of Action: An oral PROTAC (PROteolysis TArgeting Chimera) protein degrader. Unlike standard inhibitors that block the receptor, Vepdegestrant binds to the estrogen receptor (ER) and triggers the cell’s own machinery to degrade and destroy it.
Key Trial: VERITAC-2 (Phase 3).
Main Results:
- PFS Benefit in ESR1-Mutant Disease: Median PFS 5.0 vs 2.1 months vs fulvestrant (HR 0.58, P<0.001); ORR 18.6% vs 4.0%.
- Overall Population: Median PFS 3.8 vs 3.6 months (HR 0.83; P=0.07), showing strongest activity in ESR1-mutant tumors.
- Safety: Mostly grade 1–2 events; grade ≥3 AEs 23%; low discontinuation (2.9%); manageable fatigue, mild LFT elevations, and low-grade QTc prolongation.
Developer: Arvinas / Pfizer
Vepdegestrant, is an oral PROTAC estrogen receptor degrader designed to go a step beyond traditional endocrine therapy. Instead of just blocking the estrogen receptor like fulvestrant or SERDs, it recruits an E3 ligase and sends the receptor to the proteasome for destruction. The Phase 3 VERITAC-2 trial, published in The New England Journal of Medicine in May 2025, tested Vepdegestrant against fulvestrant in 624 patients with ER-positive, HER2-negative advanced breast cancer who had already received a CDK4/6 inhibitor plus endocrine therapy.

Figure From NEJM Article: Progression-free Survival as Assessed by Blinded Independent Central Review.
The clearest win was in the ESR1-mutated subgroup, which is exactly where resistance to prior endocrine therapy is most common. In these patients (about 43% of the trial), median progression-free survival was 5.0 months with Vepdegestrant versus 2.1 months with fulvestrant (hazard ratio 0.58; P<0.001), and the objective response rate jumped to 18.6% vs 4.0%. In the overall population, the difference was more modest and did not reach formal statistical significance (3.8 vs 3.6 months; HR 0.83; P=0.07), reinforcing the idea that this drug is particularly powerful in ESR1-driven disease rather than all comers.
Safety looked manageable and very “everyday clinic” for an oral endocrine agent. Most adverse events were grade 1–2; grade ≥3 events occurred in about 23% on Vepdegestrant vs 18% on fulvestrant, with low discontinuation rates (2.9% vs 0.7%). Fatigue, mild liver enzyme elevations and occasional QTc prolongation were seen but serious cardiac events were not reported, and gastrointestinal toxicity (nausea, vomiting, diarrhea) was less frequent than what has been observed with some oral SERDs.
Taken together, VERITAC-2 positions Vepdegestrant as a first-in-class PROTAC endocrine therapy with a clear niche in ESR1-mutant, ER+/HER2– advanced breast cancer. With the NDA accepted in August 2025 and a PDUFA date of June 5, 2026, it is one of the most likely near-term approvals to genuinely shift second-line endocrine practice from injections to an oral, mutation-focused degrader.
2. Darovasertib: A Vision-Saving Therapy for Uveal Melanoma
Indication: Uveal Melanoma (Neoadjuvant and Metastatic).
Mechanism of Action: A selective PKC (Protein Kinase C) inhibitor. PKC is a key driver in the GNAQ/GNA11 mutations found in approximately 90% of uveal melanoma cases.
Key Trial: Phase 2 OptimUM-09 trial
Main Results:
- Tumor Shrinkage & Eye Preservation: 83% had tumor shrinkage; ≥20% shrinkage in 54%, enabling 57% eye preservation, rising to 95% in those with ≥20% shrinkage.
- Vision Protection: 70% of plaque brachytherapy candidates had reduced predicted radiation dose, and 65% showed lower predicted risk of severe long-term vision loss; over half improved visual acuity during treatment.
- Safety & Regulatory: Generally well tolerated (grade ≥3 TRAEs 16.8%); granted FDA Breakthrough Therapy Designation as the first systemic neoadjuvant therapy with proven potential to prevent enucleation.
Developer:IDEAYA Biosciences
Darovasertib, developed by IDEAYA Biosciences in collaboration with Servier, is a potent, selective inhibitor of PKC, and is one of the most important new drugs in ocular oncology. Primary uveal melanoma is one of the few solid tumors where patients still routinely face the prospect of enucleation—surgical removal of the eye—or high-dose plaque brachytherapy that often results in major vision loss. With no approved systemic therapy for the localized disease setting, Darovasertib’s neoadjuvant strategy represents a genuine paradigm shift.
Presented at ESMO 2025 in a Proffered Paper session, the Phase 2 OptimUM-09 trial delivered some of the strongest functional-preservation data ever reported in this cancer. Across 94 evaluable patients, 83% experienced measurable tumor shrinkage and 54% achieved ≥20% shrinkage. Among patients originally recommended for enucleation, the therapy preserved the eye in 57%, and in those with ≥20% shrinkage prior to local therapy, the eye-preservation rate soared to 95%. This is unprecedented in a disease where surgical removal is often the only option.
The benefits extended beyond tumor control. In patients eligible for plaque brachytherapy, Darovasertib reduced the predicted radiation dose to critical eye structures in 70%, with two-thirds experiencing a lower predicted risk of severe vision loss at three years. Importantly, more than half of patients across both cohorts improved their visual acuity during neoadjuvant treatment—gaining an average of 17 letters (enucleation cohort) and 10 letters (brachytherapy cohort), a clinically meaningful restoration of functional vision.
Read More About Ocular Melanoma on Oncodaily
These findings, combined with a manageable safety profile—grade ≥3 treatment-related events occurred in 16.8%, with low discontinuation rates—led the FDA to grant Breakthrough Therapy Designation for Darovasertib in the neoadjuvant treatment of primary uveal melanoma. No other systemic therapy has ever demonstrated this degree of tumor control, eye preservation, and functional vision benefit in this population.
With the registrational Phase 3 OptimUM-10 trial now underway and pivotal progression-free survival data from the metastatic UM program expected in late 2025 or early 2026, Darovasertib stands out as a likely first-in-class therapy capable of redefining how uveal melanoma is treated—shifting care from organ removal to organ preservation.
3. Relacorilant: A New Option for Platinum-Resistant Ovarian Cancer
Indication: Platinum-Resistant Ovarian Cancer (PROC).
Mechanism of Action: A selective Glucocorticoid Receptor (GR) Antagonist that blocks cortisol-mediated chemotherapy resistance and restores tumor susceptibility to apoptosis.
Key Trial: ROSELLA (Phase 3).
Main Results (Updated ESMO 2025 LBA45 Data):
- PFS & OS Benefit: Relacorilant + nab-paclitaxel achieved PFS HR 0.70 (median 6.54 vs 5.52 mo) and an OS HR 0.69 with median 15.97 vs 11.50 mo.
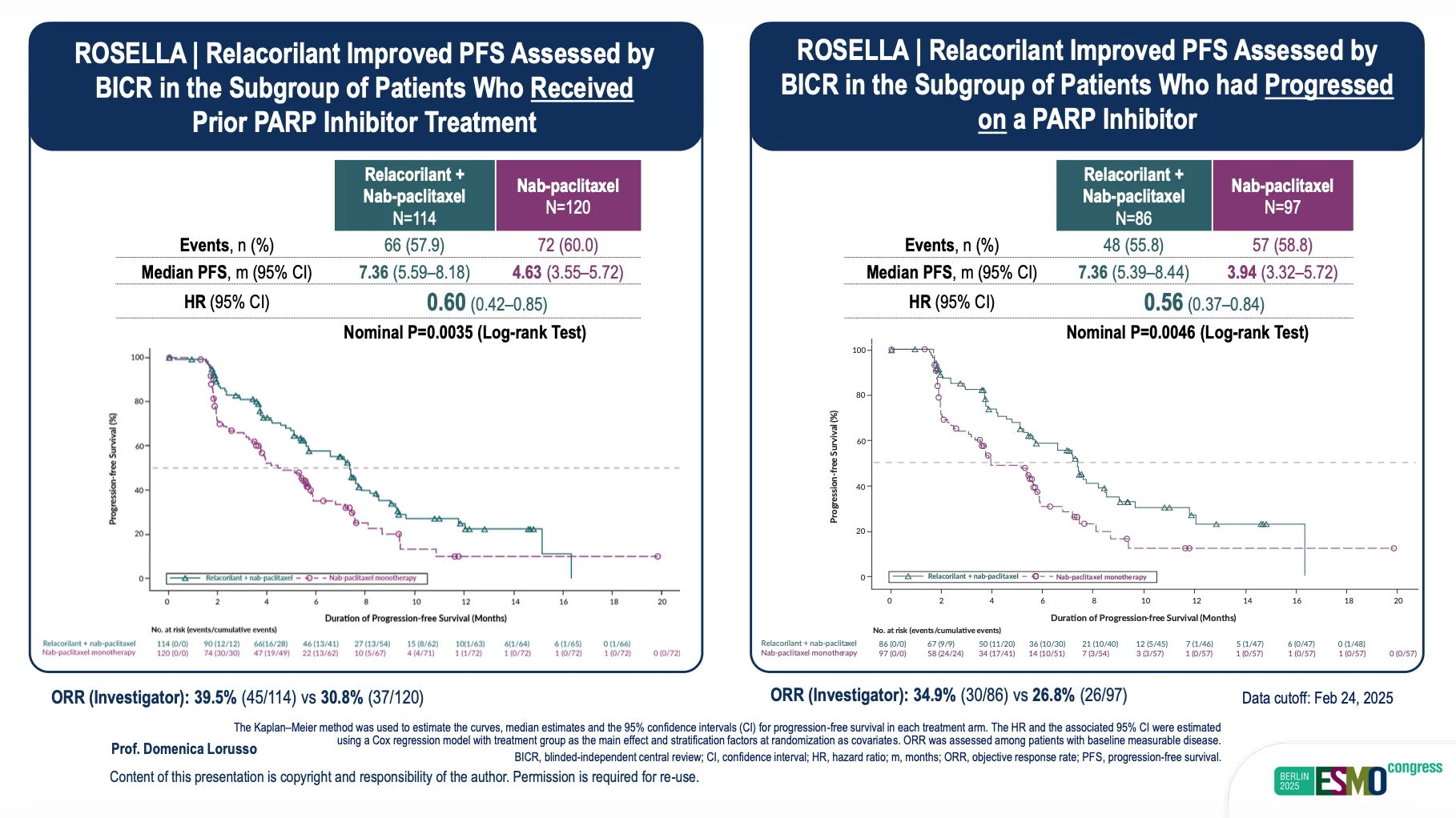
- Strong PARPi Subgroup Activity: In PARP inhibitor–exposed patients, median PFS reached 7.36 months (HR 0.60) and 7.36 months in PARPi-progressors (HR 0.56).
- Favorable Safety: Grade ≥3 and serious AEs in PARPi patients were comparable to overall ITT (71% vs 74%; 32% vs 35%), with no new safety signals.
Developer: Corcept Therapeutics.
Relacorilant is a selective glucocorticoid receptor antagonist designed to counteract cortisol-driven chemotherapy resistance—a mechanism long recognized in ovarian cancer biology but never effectively targeted. By blocking GR signaling, relacorilant enhances taxane-induced apoptosis, directly addressing a key driver of platinum-resistant ovarian cancer (PROC), where therapeutic progress has been slow for over a decade.
At ESMO 2025, the Phase 3 ROSELLA trial (LBA45) delivered evidence supporting this approach. Adding relacorilant to weekly nab-paclitaxel produced a 30% reduction in the risk of progression (HR 0.70) and a 31% reduction in the risk of death (HR 0.69), with median OS improving from 11.50 to 15.97 months. This marks the first regimen to improve both PFS and OS over weekly taxane therapy in PROC.
Presented by Domenica Lorusso at ESMO 2025 Congress
A key highlight of the updated analysis involved patients previously treated with PARP inhibitors—a subgroup known for markedly poor outcomes after PARPi progression. ROSELLA demonstrated that relacorilant can partially reverse this resistance biology. Median PFS in PARPi-exposed patients reached 7.36 months, with hazard ratios of 0.60 (prior PARPi) and 0.56 (progressed during PARPi therapy). These results compare favorably with benchmark data such as PAOLA-1, where post-PARPi patients typically show significantly shorter benefit from subsequent chemotherapy.
Critically, relacorilant achieved these improvements without adding toxicity. Safety remained consistent with nab-paclitaxel monotherapy, with no new adverse events and similar rates of grade ≥3 and serious AEs, even in the PARPi subgroup. This distinguishes relacorilant as a rare agent that meaningfully increases chemotherapy effectiveness without compromising tolerability.

Based on the strength of the ROSELLA findings, Corcept is expanding the Phase 2 BELLA program to evaluate relacorilant-containing regimens in platinum-resistant ovarian cancer, platinum-sensitive PARPi-progressors, and endometrial cancer. This expansion reflects growing confidence that GR antagonism may have broader relevance across gynecologic oncology.
With the FDA’s acceptance of the New Drug Application for PROC and a PDUFA date of July 11, 2026, relacorilant is positioned to become the first therapy to deliver dual survival improvements in this setting—and potentially the most meaningful advance in platinum-resistant ovarian cancer management in many years.
4. Daraxonrasib (RMC-6236): The First Real Pan-RAS Inhibitor
Indication: Pancreatic Cancer (and other RAS-mutated solid tumors).
Mechanism of Action: A Pan-RAS Inhibitor (RAS(ON) multi-selective inhibitor). It targets the active form of all three major RAS variants (KRAS, NRAS, HRAS), addressing a mutation historically considered “undruggable.”
Key Trial: RASolute 303 (Phase 3) and Phase 1/2 updates.
Main Results:
- High activity in PDAC: ORR 47% with monotherapy and 55% with daraxonrasib + GnP in first-line metastatic PDAC; DCR ~90%.
- Durable benefit in 2L+: ORR 29–35%, median PFS ~8 months, median OS 13–16 months, with >90% disease control.
- Favorable safety: Mostly rash and mild GI events; no treatment discontinuations due to toxicity; dose intensity >80%.
Developer: Revolution Medicines
Daraxonrasib (RMC-6236), represents one of the most advanced efforts to target RAS—often described as the most difficult oncogenic driver in solid tumors. Unlike mutation-specific drugs such as KRAS G12C inhibitors, daraxonrasib is a multi-selective RAS(ON) inhibitor, designed to suppress a broad spectrum of pathogenic RAS variants including G12X, G13X, and Q61X, which together drive the overwhelming majority of pancreatic ductal adenocarcinomas (PDAC).
Pancreatic cancer remains a malignancy with extremely poor survival outcomes, where median survival in the metastatic setting is measured in months and chemotherapy remains the only systemic standard. Against this backdrop, the breadth and consistency of daraxonrasib’s activity in 2025 generated significant clinical optimism.
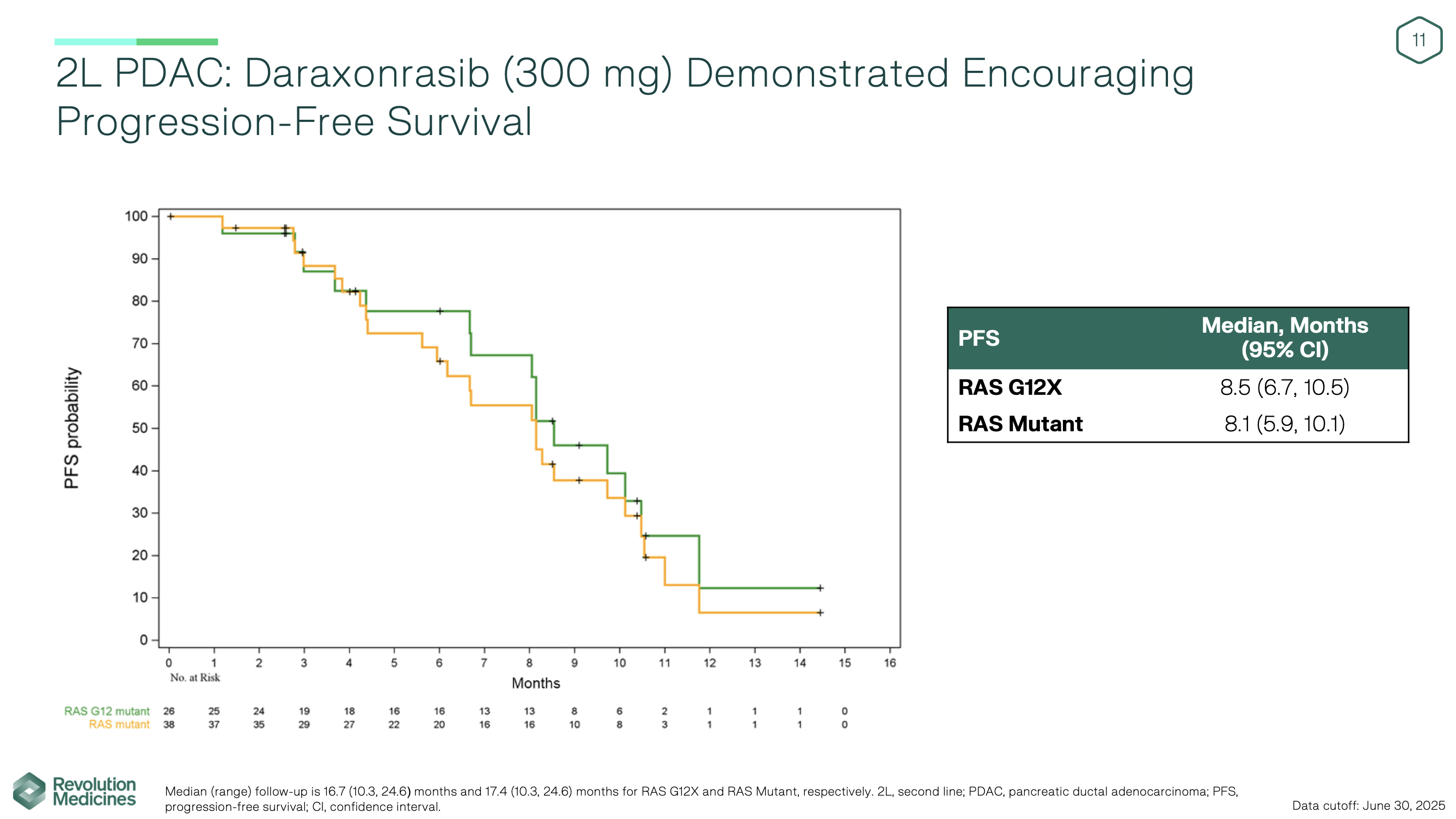
In the second-line metastatic PDAC cohort, long-term follow-up showed durable activity rarely seen in this setting. At the 300 mg daily dose, daraxonrasib achieved ORR 29–35%, disease-control rates over 90%, and median PFS of 8.1–8.5 months. Median OS reached 13.1–15.6 months, with a median follow-up of approximately 17 months. The safety profile remained manageable: rash and mucositis/stomatitis were the most common toxicities, grade ≥3 events occurred in about one-third of patients, and importantly, no patients discontinued therapy due to toxicity, with dose intensity maintained at 86%.
Early first-line metastatic PDAC data further strengthened the signal. Daraxonrasib monotherapy yielded a 47% response rate, and the combination of daraxonrasib with gemcitabine/nab-paclitaxel delivered an ORR of 55%, with ~90% disease control and no unexpected safety concerns. Mean dose intensity remained above 80%, indicating good tolerability even in combination regimens. These response levels exceed historical expectations for first-line chemotherapy in PDAC and suggest that RAS-directed therapy may be viable even in untreated disease.
These findings support the ongoing RASolute 303 Phase 3 trial, which is enrolling first-line metastatic PDAC patients into three arms—daraxonrasib monotherapy, daraxonrasib + GnP, and GnP alone—with PFS and OS as dual primary endpoints. In parallel, the RASolute 302 Phase 3 trial is evaluating daraxonrasib monotherapy in second-line PDAC, and an additional Phase 3 study is planned in the adjuvant setting.
In October 2025, the FDA granted Orphan Drug Designation to daraxonrasib for pancreatic cancer, reflecting both the severity of the disease and the strength of emerging clinical evidence.
If the ongoing Phase 3 trials confirm the activity seen in early studies, daraxonrasib could become the first broadly active RAS inhibitor for pancreatic cancer—potentially redefining the therapeutic landscape of a disease historically dominated by cytotoxic therapy alone.
Read More About RASolute Trial on OncoDaily GI
Zanzalintinib: A New Potetial Standard of Care for Advanced Colorectal Cancer?
Indication: Refractory Colorectal Cancer (CRC).
Mechanism of Action: A next-generation Multi-kinase Inhibitor targeting VEGFR, MET, and the TAM family kinases (Tyro3, Axl, Mer), which are involved in tumor growth and immune evasion.
Key Trial: STELLAR-303 (Phase 3).
Main Results:
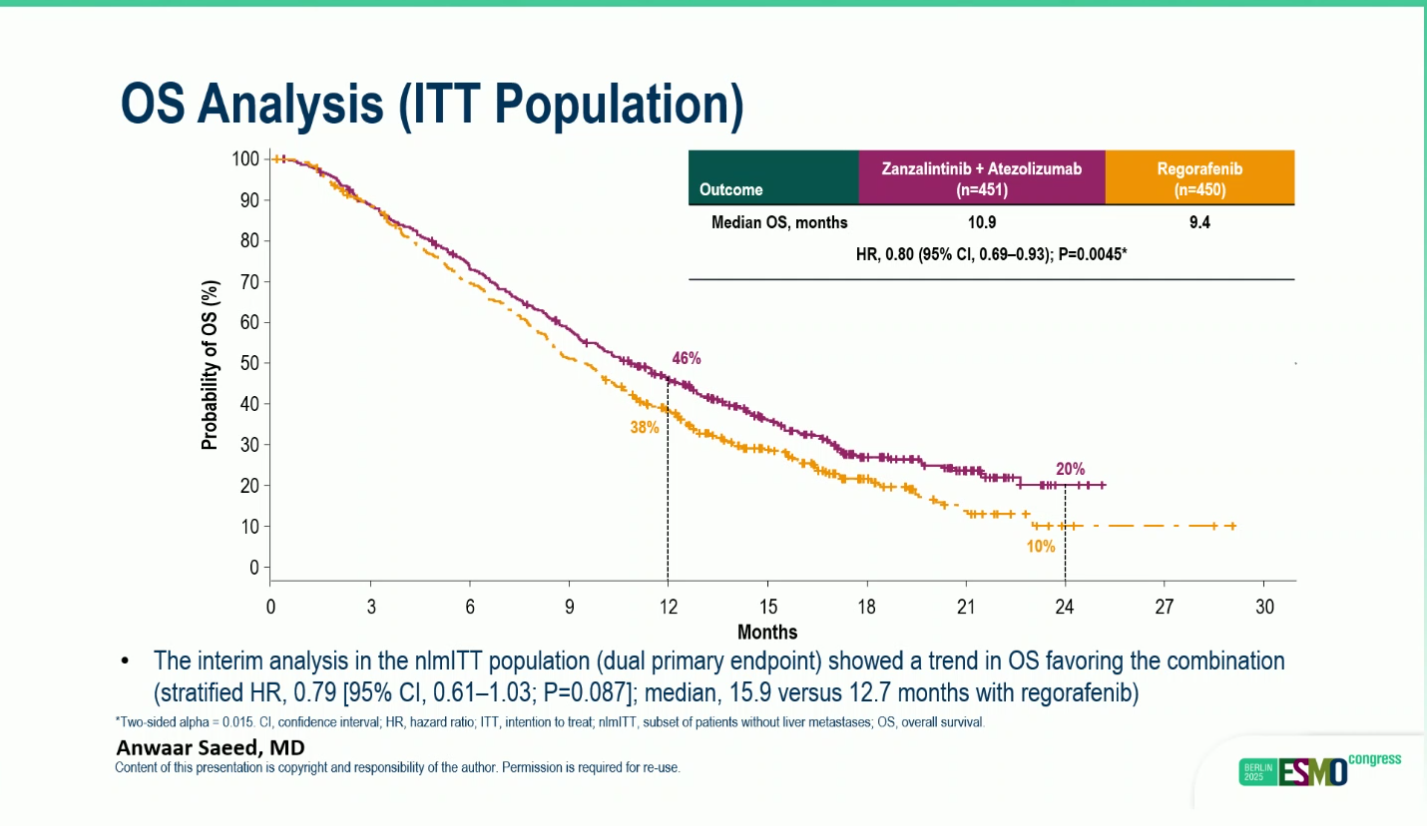
- OS Benefit: Median OS 10.9 vs 9.4 months vs regorafenib (HR 0.80, p=0.0045) in refractory MSS metastatic colorectal cancer.
- PFS Benefit: Median PFS 3.7 vs 2.0 months (HR 0.68), with consistent benefit across all subgroups—including patients with or without liver metastases.
- Manageable Safety: Grade 3/4 TRAEs in 59%, mainly hypertension (15%), fatigue (6%), diarrhea (6%), and proteinuria (6%); no new safety signals.
Presented by Anwaar Saeed at ESMO 2025 Congress
The phase III STELLAR-303 trial delivered the first randomized evidence that a multitargeted tyrosine kinase inhibitor combined with immunotherapy can extend survival in microsatellite-stable metastatic colorectal cancer (MSS mCRC), a population historically resistant to checkpoint inhibition. In this global study of 901 previously treated patients, zanzalintinib plus atezolizumab achieved a statistically significant improvement in overall survival compared with regorafenib, the current standard of care. Median OS reached 10.9 months with the combination versus 9.4 monthswith regorafenib (HR 0.80; p=0.0045), and this advantage was consistent across all predefined subgroups, including patients with the traditionally immune-refractory profile of active liver metastases.
Progression-free survival also favored the combination, with a median PFS of 3.7 months compared with 2.0 months for regorafenib (HR 0.68). Although the absolute magnitude of benefit was modest, the findings remain clinically meaningful in the highly refractory MSS setting. Importantly, the safety profile aligned with the known effects of VEGF/MET/TAM pathway inhibition. Grade 3–4 treatment-related toxicities occurred in 59% of patients receiving the combination, most commonly hypertension, diarrhea, fatigue, and proteinuria, but no new or unexpected safety signals emerged.

Read More About STELLAR-303 Trial on OncoDaily
Overall, STELLAR-303 positions zanzalintinib plus atezolizumab as a potential new therapeutic approach for patients with refractory MSS mCRC, supporting further biomarker-driven evaluation and ongoing final OS analyses in patients without liver metastases.
6. GSK5764227 (HS-20093): A Long-Awaited Breakthrough B7-H3 ADC for Sarcomas
Indication: Relapsed/Refractory Osteosarcoma and Soft Tissue Sarcoma (STS).
Mechanism of Action: A B7-H3–targeted antibody-drug conjugate (ADC) carrying a topoisomerase I inhibitor payload.
Key Trial: ARTEMIS-001 (Phase 1/2).
Main Results:
- Osteosarcoma: ORR 20%, DCR 86% in heavily pretreated disease (historical ORR <5% with chemotherapy).
- Soft Tissue Sarcoma: ORR 23%, DCR 92%, with responses across multiple STS subtypes.
- Safety: Manageable toxicity profile with no unexpected safety signals; consistent with topo-I ADC class.
Developer:
GSK5764227 (formerly HS-20093) is a first-in-class B7-H3–directed antibody–drug conjugate (ADC) incorporating a topoisomerase I inhibitor payload, designed to target B7-H3–expressing solid tumors. B7-H3 is highly expressed in osteosarcoma and multiple soft tissue sarcoma (STS) subtypes, making it an attractive therapeutic target in diseases with extremely limited systemic therapy responsiveness. Following GSK’s acquisition of Hansoh Bio’s ADC platform, GSK5764227 has advanced into global development as a next-generation cytotoxic ADC for sarcomas.
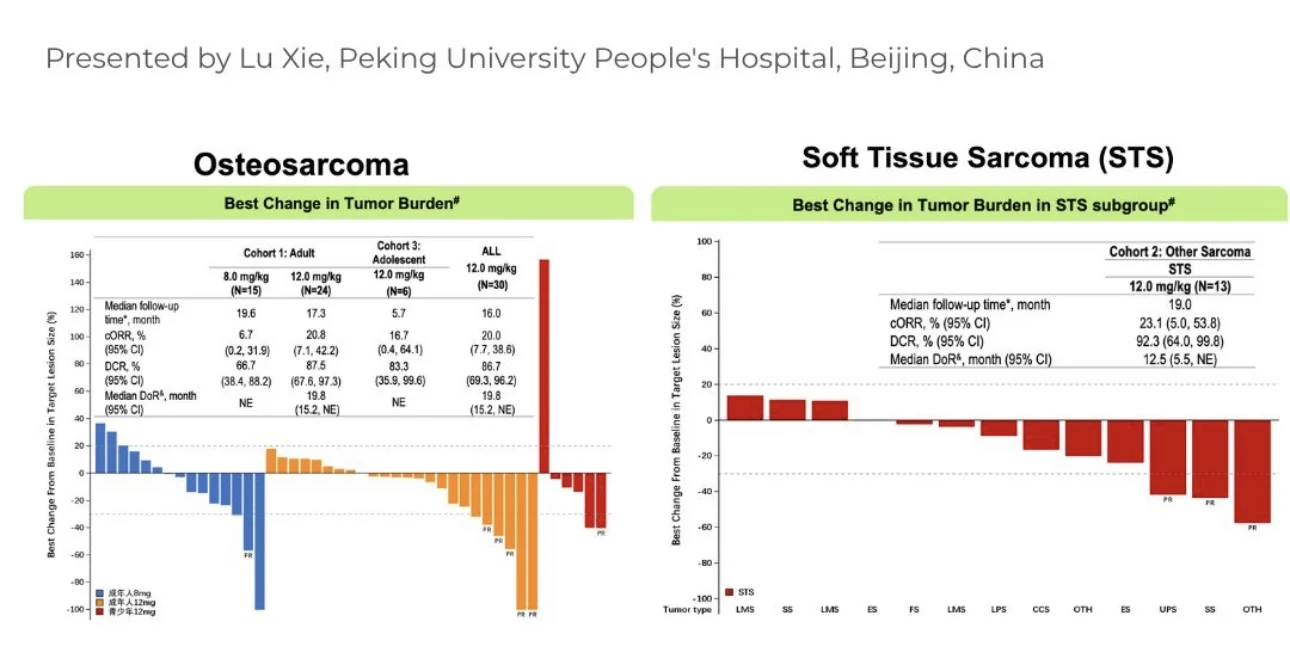
Updated results from the ARTEMIS-001 Phase 1/2 study were presented at ESMO 2025 and demonstrated clinically meaningful activity in heavily pretreated sarcoma populations. In relapsed/refractory osteosarcoma, GSK5764227 achieved an Objective Response Rate (ORR) of 20% and Disease Control Rate (DCR) of 86%, markedly exceeding the historical ORR of <5% associated with salvage chemotherapy. In the soft tissue sarcoma cohort, ORR reached 23%with a DCR of 92%, with responses observed across multiple sarcoma subtypes.
The safety profile was consistent with topoisomerase-based ADCs; treatment-related adverse events were manageable, and no new safety signals emerged. Importantly, the drug demonstrated durable tumor shrinkage and disease stabilization in a population with few alternative treatment options and poor prognoses.
Collectively, the ARTEMIS-001 data position GSK5764227 as a potentially transformative therapeutic candidate in bone and soft tissue sarcomas, offering one of the most substantial improvements in response rates seen in this disease area in years. Expansion into later-phase studies is anticipated.
7. Anbenitamab (KN026): A Next-Generation HER2 Bispecific Antibody
Indication: HER2-Positive Gastric Cancer (GC) and GEJ
Mechanism of Action: A bispecific HER2 antibody binding two non-overlapping HER2 epitopes (ECD2 + ECD4), enhancing HER2 blockade, receptor internalization, and ADCC.
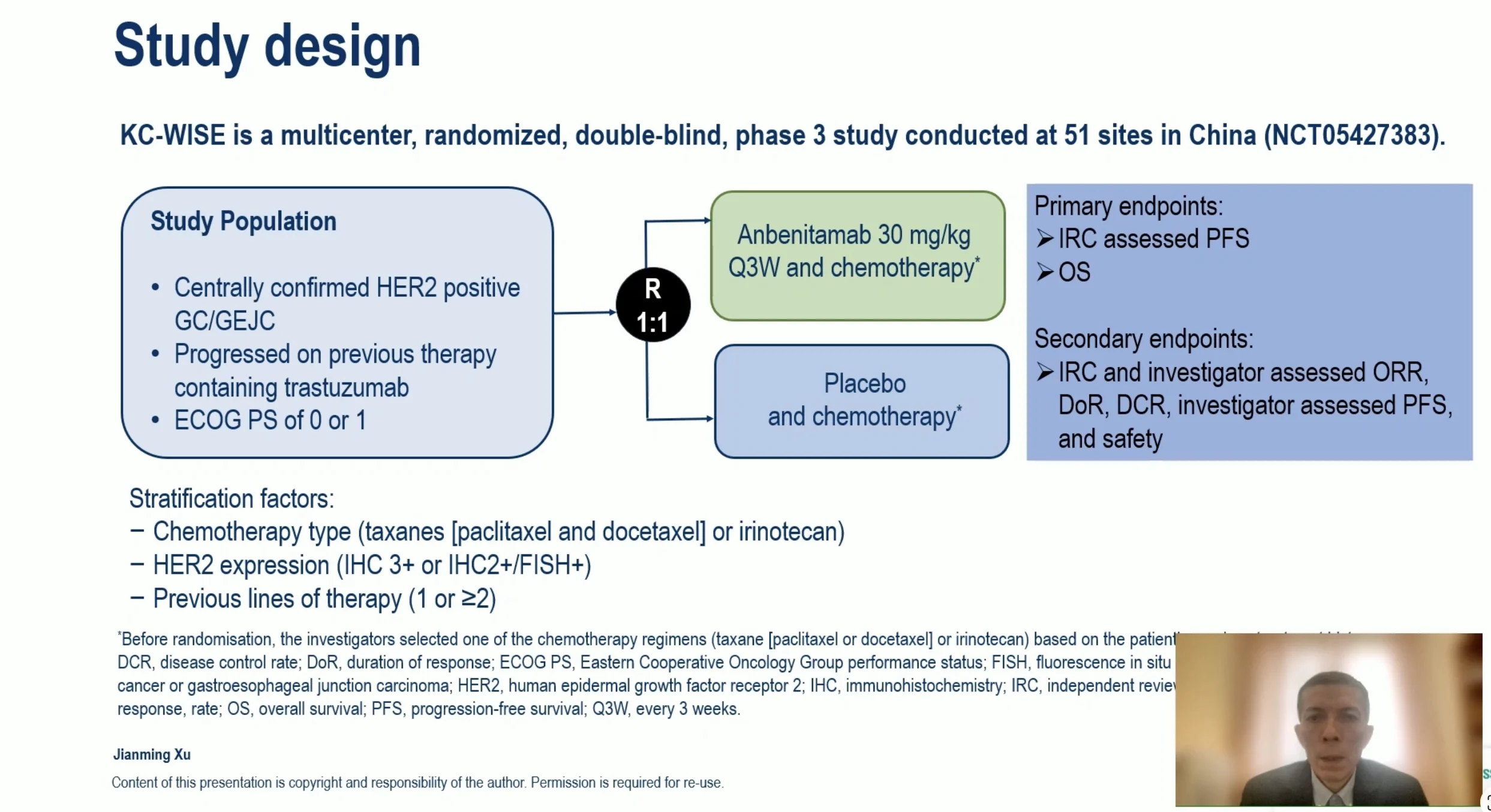
Key Trial: KC-WISE (KN026-001, Phase 3).
Main Results:
- PFS Benefit: Median PFS 7.1 vs 2.7 months vs chemotherapy (HR 0.25, p=5.44×10⁻¹²), representing a 75% reduction in risk of progression or death.
- OS Benefit: Median OS 19.6 vs 11.5 months (HR 0.29, p=1.56×10⁻⁶), a 71% reduction in risk of death; OS not yet mature.
- High Response Rates: ORR 55.8% vs 10.8%; DCR 80% vs 41.9%; median DoR 8.2 vs 2.9 months.
- Manageable Safety: Grade ≥3 TEAEs 60.6%, primarily neutropenia, leukopenia, anemia, diarrhea, and asthenia; low cardiotoxicity (3.2%), comparable to control.
Developer: Alphamab Oncology
The first interim analysis of the Phase III KC-WISE (KN026-001) trial presented at the ESMO Congress 2025 demonstrated that anbenitamab (KN026) combined with chemotherapy provides a significant and clinically meaningful benefit in previously treated HER2-positive gastric and gastroesophageal junction (GC/GEJ) cancers after progression on trastuzumab-containing regimens.
In this randomized, double-arm study, patients with HER2-positive GC/GEJ who experienced disease progression following trastuzumab-based therapy were assigned to receive anbenitamab + chemotherapy or placebo + chemotherapy. Baseline characteristics were well-balanced, with most patients presenting with ECOG PS 1 and stage IVB disease. The median follow-up was approximately 9.7 months in both arms.
Presented By Jianming Xu, ESMO 2025
Treatment with anbenitamab yielded a statistically significant improvement in progression-free survival (PFS) compared with chemotherapy alone. Median PFS was 7.1 months in the anbenitamab arm versus 2.7 months in the control arm, corresponding to a hazard ratio (HR) of 0.25 (P = 5.44 × 10⁻¹²), indicating a 75% reduction in risk of disease progression or death. Overall survival (OS) also favored anbenitamab, with a median OS of 19.6 months (not yet mature) versus 11.5 months in the control arm, representing an HR of 0.29 (P = 1.56 × 10⁻⁶) and a 71% reduction in risk of death. Both primary endpoints of the trial—PFS and OS—were met with high statistical robustness.
Objective response outcomes were similarly improved. The ORR was 55.8% with anbenitamab compared with 10.8% in the control group, and the disease control rate (DCR) reached 80.0% vs. 41.9%, respectively. The median duration of response (DoR) was 8.2 months for anbenitamab versus 2.9 months for control therapy, indicating more durable benefit.
Safety findings showed that anbenitamab plus chemotherapy was tolerable and consistent with expected HER2-directed therapy toxicities. Grade ≥3 TEAEs occurred in 60.6% of patients receiving anbenitamab versus 51.6% in the control group, with neutropenia, leukopenia, anemia, diarrhea, and asthenia being the most common high-grade events. Importantly, cardiotoxicity was low (3.2%) and comparable between arms, despite the HER2-targeting mechanism.

Presented By Jianming Xu, ESMO 2025
These interim data indicate that anbenitamab significantly improves PFS, OS, ORR, and durability of response in trastuzumab-pretreated HER2-positive GC/GEJ, positioning it as a strong potential contender for second-line and later-line therapy. The magnitude of benefit compares favorably with results from DESTINY-Gastric04 (trastuzumab deruxtecan), suggesting possible efficacy and safety advantages. Based on these findings, additional trials are planned to expand anbenitamab development into first-line and perioperative settings.
8. iNKT Cell Therapy: An “Off-the-shelf” cell therapy with Transformative Potential
Indication: Refractory Solid Tumors (specifically Metastatic Germ Cell Tumors and Gastric Cancer).
Mechanism of Action: An allogeneic, “off-the-shelf” invariant Natural Killer T (iNKT) cell therapy. It targets CD1d (a lipid-presenting molecule on tumor cells) to trigger a dual attack: direct tumor lysis via perforin/granzyme and rapid modulation of the suppressive tumor microenvironment through the release of cytokines (like IFN-gamma) to recruit host immune cells.
Key Trial: Phase I Trial in Patients With Relapsed/ Refractory Solid Tumors
Main Results:
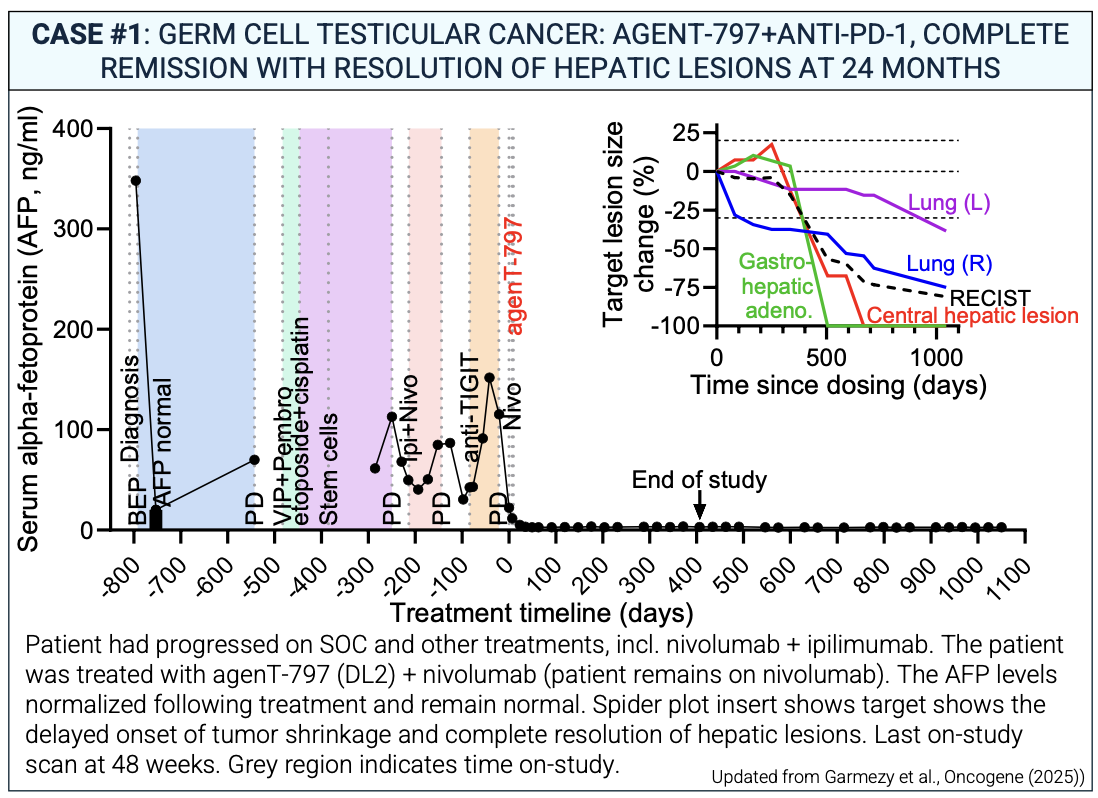
- Germ Cell Tumor Breakthrough: Achieved a confirmed Complete Remission (CR) in a patient with metastatic disease refractory to 7 prior lines of therapy (including high-dose chemotherapy and stem cell transplant), as published in Oncogene (July 2025).
- Gastric Cancer Activity: Demonstrated a Disease Control Rate (DCR) of ~70% in heavily pre-treated gastric cancer patients, showing efficacy in tumors typically resistant to standard checkpoint inhibitors.
- Safety Profile: Highly tolerable with no Graft-versus-Host Disease (GvHD) or severe neurotoxicity observed, validating its potential as a scalable therapy that does not require HLA matching.
Updated Phase 1 data presented at SITC 2025 highlight agenT-797 as one of the most promising entrants in next-generation, off-the-shelf cell therapy. In patients with PD-1–refractory, heavily pretreated solid tumors, the combination of agenT-797 with anti-PD-1 produced durable and meaningful clinical benefit, with a median overall survival approaching 23 months—a striking result in a population with historically poor outcomes. Several patients achieved deep, long-lasting remissions, including a complete and sustained response beyond two years in metastatic germ-cell/testicular cancer, and prolonged disease control in gastric cancer, thymoma, cholangiocarcinoma, renal cancer, and adenoid cystic carcinoma.
Presented by Ben Garmezy, at SITC 2025
Mechanistic analyses reveal why: agenT-797 functions not only through direct cytotoxicity but through immune re-orchestration, activating dendritic cells, reversing macrophage suppression, rescuing exhausted T cells, and enhancing CD8⁺ and NK-cell infiltration into tumors. The therapy maintained an exceptionally clean safety profile, with no dose-limiting toxicities, no high-grade CRS, and no neurotoxicity, underscoring its suitability for combination regimens and repeat dosing.
Read More About iNKT on OncoDaily IO
Together, these findings position agenT-797 as a first-in-class allo-iNKT cell therapy capable of restoring immune responsiveness in checkpoint-resistant solid tumors and advancing a new therapeutic paradigm for immune-cold cancers.
9. Pasritamig (JNJ-78278343): A New Bispecific Weapon in Prostate Cancer
Indication: Metastatic Castration-Resistant Prostate Cancer (mCRPC).
Mechanism of Action: A first-in-class Bispecific T-cell Engager (BiTE) targeting Human Kallikrein-related Peptidase 2 (KLK2).
Key Trial: Phase 1 First-in-Human Study (NCT04898634).
Main Results:
- PSA Response: In the Recommended Phase 2 Dose (RP2D) group, 42.4% of patients achieved a PSA50 response (≥50% reduction in PSA levels). This occurred even in patients who had failed prior potent androgen receptor inhibitors and taxane chemotherapy.
- PFS Benefit: The median Radiographic Progression-Free Survival (rPFS) was 7.9 months (95% CI 2.9–NE).
- Manageable Safety: Low CRS: Cytokine Release Syndrome (CRS) occurred in only 8.9% of patients, and importantly, all cases were Grade 1 (mild). No Neurotoxicity: No ICANS (Immune Effector Cell-Associated Neurotoxicity Syndrome) was observed.
Developer: Janssen Research & Development (a Johnson & Johnson company)
Pasritamig (JNJ-78278343) is a first-in-class bispecific T-cell–engaging antibody directed against human kallikrein-2 (KLK2), a prostate-specific serine protease highly expressed in metastatic castration-resistant prostate cancer (mCRPC). Its design enables selective T-cell redirection against KLK2-expressing tumor cells while minimizing off-tumor targeting, a long-standing limitation of T-cell therapies in prostate cancer. Based on emerging clinical activity and favorable tolerability, the FDA granted Fast Track designation in 2025, facilitating accelerated development and eligibility for priority review.
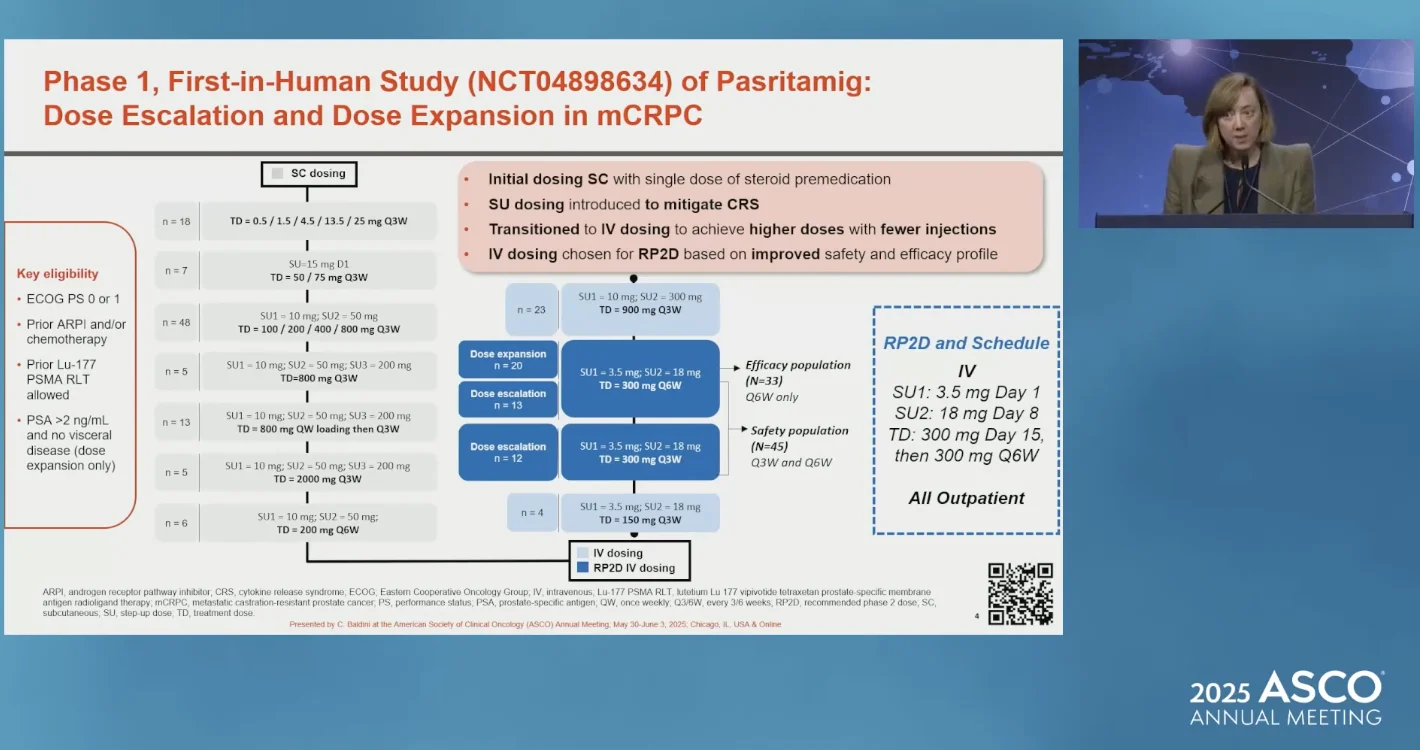
Presented by Capucine Baldini at ASCO 2025
Phase 1 results (NCT04898634) presented at ASCO and expanded at ESMO 2025 included 174 patients with mCRPC who had received ≥1 prior systemic therapies. Pasritamig displayed manageable toxicity, with 82.2% experiencing a treatment-related adverse event (TRAE), mostly low-grade. Grade ≥3 TRAEs occurred in only 9.2%, and CRS—typically a major concern with bispecifics—occurred in <10%, all Grade 1. The most frequent TRAEs at the recommended Phase 2 dose (RP2D) were infusion-related reactions (22.2%), fatigue (15.6%), and low-grade CRS (8.9%).
The RP2D regimen was established as:
- SUI 3.5 mg Day 1, SU2 18 mg Day 8, then
- 300 mg Day 15, followed by 300 mg every 6 weeks (Q6W) intravenously.
This schedule produced the most favorable balance between pharmacokinetics, immunologic engagement, and tolerability. In this RP2D cohort, the median radiographic PFS was 7.9 months (95% CI, 2.9–NE), and 42.4% of patients achieved a ≥50% PSA decline, an early marker of antitumor activity in mCRPC. Responses were observed across patients with extensive prior therapies and molecularly heterogeneous disease.

Presented by Capucine Baldini at ESMO 2025
At ESMO 2025, investigators presented a detailed translational analysis comparing dosing intervals. Weekly and Q3W dosing schedules were associated with rising PSA values and evidence of T-cell exhaustion. In contrast, Q6W dosing preserved a reprogrammable progenitor CD8⁺ T-cell compartment, characterized by lower expression of activated caspase-3 and γH2AX in peripheral blood mononuclear cells (n=186), indicating reduced activation-induced cell death (AICD). This immunologic preservation correlated with superior clinical activity: 44% of patients on the Q6W schedule achieved a complete response at any time, compared with 33% on Q3W.
Investigators emphasized that the degree of progenitor T-cell maintenance strongly correlated with PSA50 responses, independent of dose level, providing mechanistic validation that KLK2-directed T-cell redirection can produce clinically actionable immunity in prostate cancer.
Collectively, Phase 1 clinical and correlative data support pasritamig as a potentially impactful therapeutic strategy for mCRPC. It is the first agent to successfully leverage KLK2 as a T-cell–engaging target, with a safety profile compatible with outpatient delivery and enough preliminary efficacy to justify accelerated development. Upcoming Phase 2 studies are expected to further define its role within the evolving immunotherapy landscape for advanced prostate cancer.
10. Iza-bren (Izalontamab Brengitecan): An EGFR×HER3 ADC for NSCLC, SCLC and Nasopharyngeal Cancer
Indication: EGFR-mutant NSCLC after third-generation EGFR TKI and platinum chemotherapy; recurrent/metastatic nasopharyngeal carcinoma (R/M-NPC) after ≥2l; relapsed/refractory small cell lung cancer (SCLC); NSCLC with non-classical oncogenic drivers.
Mechanism of Action: A first-in-class EGFR×HER3 bispecific antibody–drug conjugate linked to a topoisomerase-I inhibitor payload (Ed-04)
Key Trials: BL-B01D1-101/102 (Phase I/Ib); BL-B01D1-303 (Phase III NPC).
Main Results:
- NPC (Phase III): cORR 54.6% vs 27.0% with chemotherapy; median PFS 8.38 vs 4.34 months (HR 0.44); DoR 8.51 vs 4.76 months. OS immature. Grade ≥3 TRAEs 79.9%, predominantly hematologic, manageable with supportive care.
- NSCLC (Phase Ib): ORR 45.6%, cORR 35.3%, DCR 82.4%, median PFS 6.7 months across multiple oncogenic drivers. EGFR exon20ins and HER2 cohorts showed highest activity (cORR ~67%, DCR 100%). Only one low-grade ILD event.
- SCLC (Phase I): ORR 55.2%, confirmed ORR 44.8%, median PFS 4.0 months, OS 12.0 months. In patients with only one prior line of chemo-immunotherapy: ORR 80%, cORR 75%, median PFS 6.9 months, OS 15.1 months.
Safety: Class-typical topo-I ADC profile with predominantly hematologic toxicity
Developer: SystImmune and Bristol Myers Squibb (BMS)
Iza-bren (izalontamab brengitecan) is emerging as one of the most versatile next-generation ADCs in solid tumors, not because it targets a single niche mutation, but because it leverages a dual-receptor strategy that is relevant across multiple epithelial cancers. EGFR and HER3 form a powerful signaling pair: EGFR drives proliferation, while HER3 acts as an amplifier of ligand-mediated PI3K/AKT signaling and a key partner in resistance to targeted therapy. By co-targeting both and delivering a topoisomerase-I payload, iza-bren is designed to hit the signaling architecture and the DNA simultaneously.
The first indication where iza-bren looks truly practice-changing is recurrent/metastatic nasopharyngeal carcinoma. In BL-B01D1-303, a heavily pretreated R/M-NPC population (≥2 prior chemo lines, prior PD-(L)1) was randomized to iza-bren or physician’s-choice capecitabine, gemcitabine, or docetaxel. The interim analysis, presented as a late-breaking oral at ESMO 2025 and published in The Lancet, showed that iza-bren more than doubled confirmed ORR (54.6% vs 27.0%) and nearly doubled median PFS (8.38 vs 4.34 months; HR 0.44), with responses lasting almost twice as long (median DoR 8.51 vs 4.76 months). OS is not yet mature, but the magnitude of PFS and response benefit alone strongly suggests that this ADC can outperform late-line chemotherapy in a space with historically modest options.
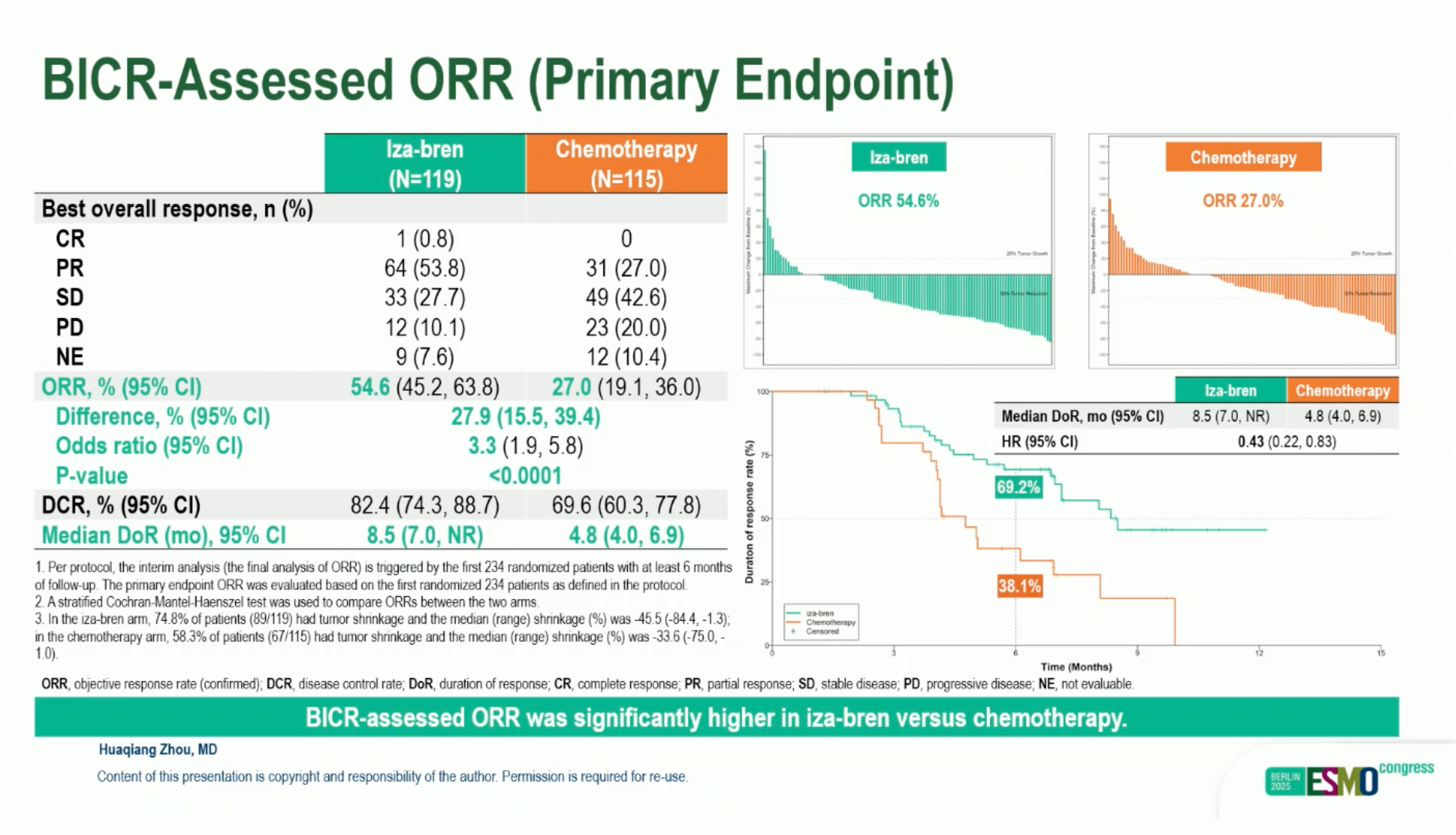
Presented by Huaqiang Zhou at ESMO 2025
Toxicity in BL-B01D1-303 was dominated by myelosuppression, reflecting the potent topo-I payload, with grade ≥3 TRAEs in about 80% of patients versus 62% with chemotherapy. However, treatment discontinuation rates and non-hematologic toxicities were acceptable, and no new safety signals emerged compared with earlier studies.
Beyond NPC, iza-bren has generated a surprisingly broad signal in lung cancer. In the phase Ib NSCLC expansion presented at ASCO 2025, patients were enrolled by genotype rather than by histology—EGFR exon 20 insertions, atypical EGFR mutations, HER2, ALK/ROS1/RET fusions, KRAS (including G12C), BRAF, MET exon 14, NTRK, and SMARCA4. Most had progressed on standard targeted therapy when available and had received ≤1 prior line of chemotherapy.
At the RP2D of 2.5 mg/kg D1D8 Q3W, the overall ORR was ~46%, with a cORR of 35% and DCR above 80%; median PFS was 6.7 months. The most striking activity was seen in EGFR exon 20 insertion and HER2-mutant disease, where confirmed response rates exceeded 50–65% and disease control was near-universal, with median PFS not reached in some cohorts at the time of reporting.
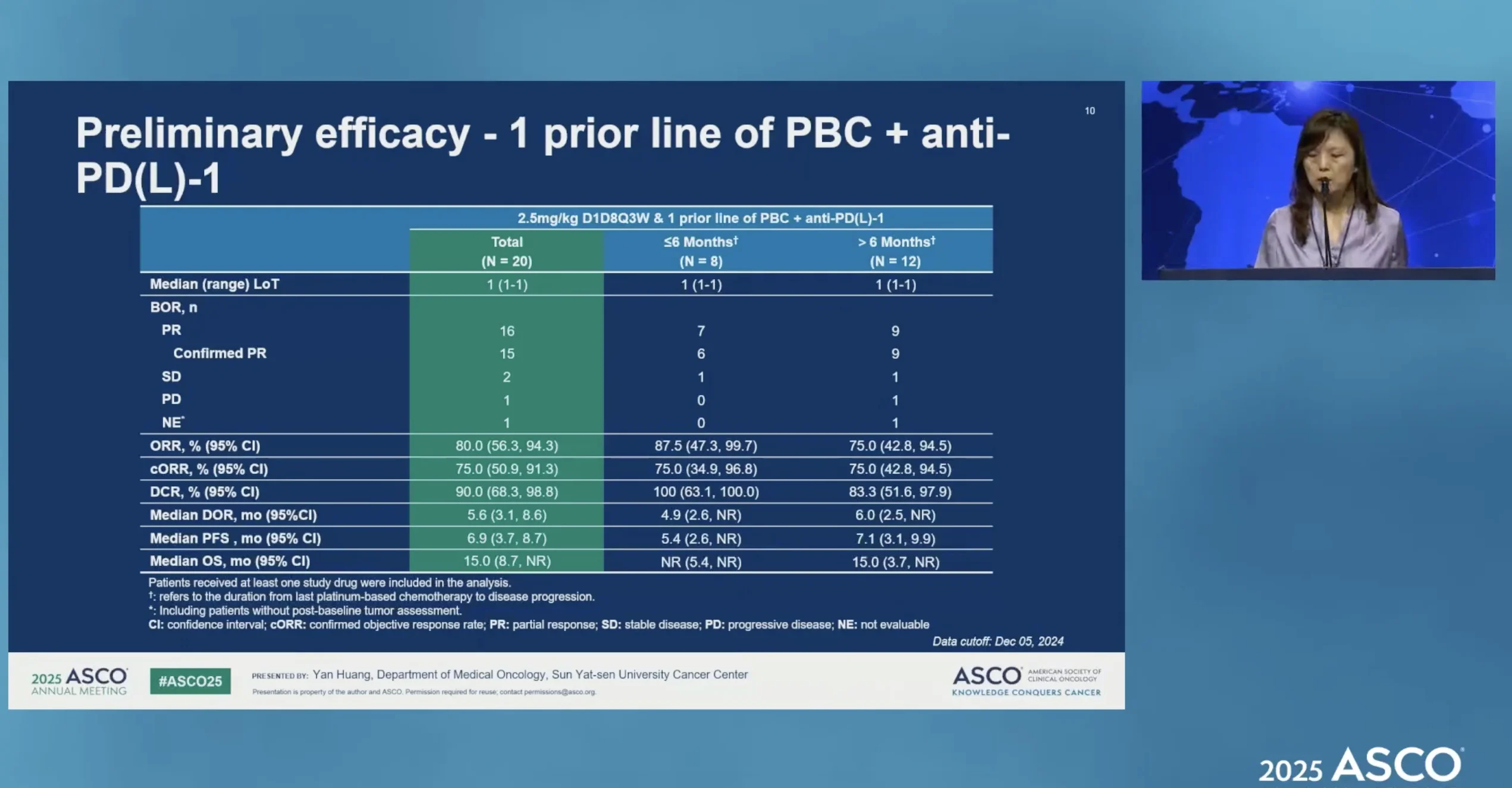
Presented by Yan Huang at ASCO 2025
In SCLC, where meaningful progress has been rare, the phase I BL-B01D1-101 results are striking. Among 58 previously treated patients, iza-bren achieved an ORR of 55.2% (confirmed 44.8%), with median PFS 4.0 months and OS 12.0 months. Activity was even stronger in patients treated after only one prior line of PD-(L)1 plus platinum: ORR reached 80%, confirmed ORR 75%, and median PFS and OS were 6.9 and 15.1 months. These data justify the ongoing phase III trial in SCLC after one prior PD-(L)1 + platinum regimen (NCT06500026)
Why “Not Yet Approved” Still Matters Now
In oncology, “not yet approved” is not a technical footnote – it is the whole context. These medicines are still investigational: their safety and efficacy are being defined in controlled trials, not yet in everyday practice. Early response rates and PFS curves can look extraordinary, but until regulators review mature data in larger, more diverse populations, these drugs remain accessible mainly through clinical trials or carefully selected compassionate use. For patients and clinicians, that means hope, but not yet a standard of care.
The therapies highlighted here were selected by the OncoDaily Research & Intelligence Editorial Team based solely on the strength of their clinical data to date – agents showing meaningful activity in difficult cancers, innovative mechanisms, and the potential to shift treatment expectations if pivotal trials confirm their promise.
Together, this list of 10 most promising cancer drugs not yet approved, reflect the direction in which cancer medicine is moving—and remind us that tomorrow’s breakthroughs are already being written today.
Written by Amalya Sargsyan, MD, MSc, OncoDaily Research & Intelligence Editorial Team


