The STELLAR-303 trial (NCT05425940), presented by Dr. Anwaar Saeed during the ESMO Congress 2025, delivered updates in colorectal cancer research. The study demonstrated that zanzalintinib (zanza), a next-generation multi-kinase inhibitor, when combined with the PD-L1 inhibitor atezolizumab (atezo), achieved a statistically significant and clinically meaningful improvement in overall survival (OS) compared with regorafenib, the current standard of care in the salvage setting. This marks a major step forward for patients with microsatellite-stable (MSS) metastatic colorectal cancer (mCRC)—a population historically resistant to immunotherapy.
Background
Patients with microsatellite-stable metastatic colorectal cancer (MSS mCRC) face a major therapeutic challenge, as standard chemotherapy and targeted regimens often yield diminishing returns after multiple lines of therapy, and immune checkpoint inhibitors (ICIs)—which have revolutionized treatment for several other malignancies—have shown minimal activity in this subgroup. The immune resistance of MSS tumors is largely attributed to their “cold” tumor microenvironment, characterized by low tumor mutational burden, scarce T-cell infiltration, and immunosuppressive signaling pathways that hinder effective antitumor immune responses.
Zanzalintinib (XL092) is a cutting-edge, multi-targeted tyrosine kinase inhibitor primarily directed against VEGFR, with additional immunomodulatory actions. This comprehensive review summarizes both preclinical and early clinical findings, and outlines future prospects for zanzalintinib—especially when combined with immune checkpoint inhibitors (ICIs).
Preclinical insights suggest that zanzalintinib’s inhibition of MET and TAM kinases may enhance immune responses, supporting its synergy with ICIs. Early clinical trials, including a phase 1 dose-escalation study, have demonstrated that pairing zanzalintinib with an anti–PD-L1 agent is well tolerated, without unexpected toxicities.
Study Design
The trial enrolled 901 patients with relapsed or refractory MSS mCRC, randomized 1:1 to receive either zanzalintinib (100 mg once daily) plus atezolizumab (1200 mg every 3 weeks) or regorafenib (160 mg once daily on days 1–21 of each 28-day cycle). The dual primary endpoints were overall survival (OS) in the intention-to-treat (ITT) population and in the subset of patients without liver metastases (nlmITT).
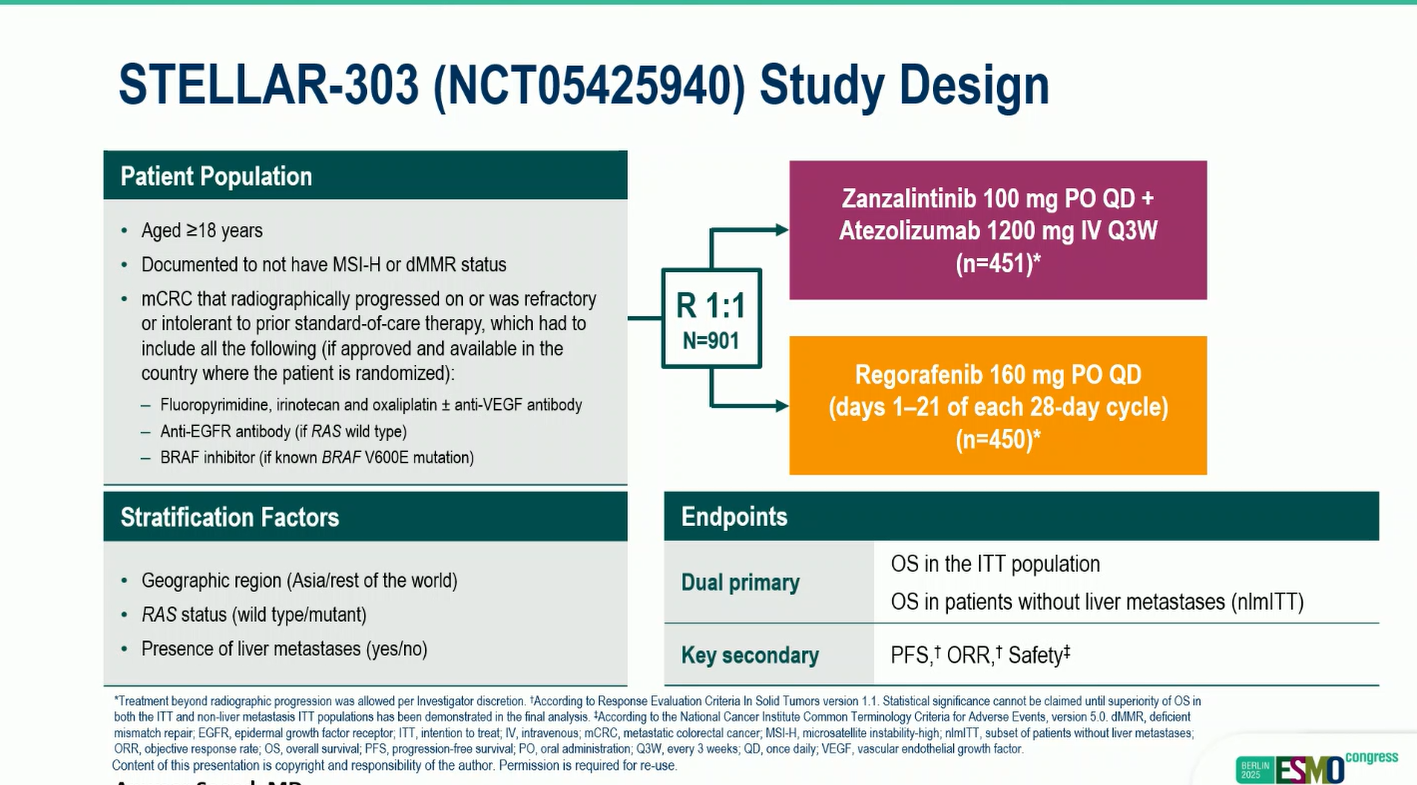
Key Findings
At a median follow-up of 18 months, the combination of zanza + atezo significantly improved outcomes over regorafenib:
- Median OS: 10.9 vs 9.4 months
- Hazard ratio (HR): 0.80 (95% CI, 0.69–0.93; P = 0.0045)
- OS at 12 mounts 46% vs 38% and at 24 mounts 20% vs 10%
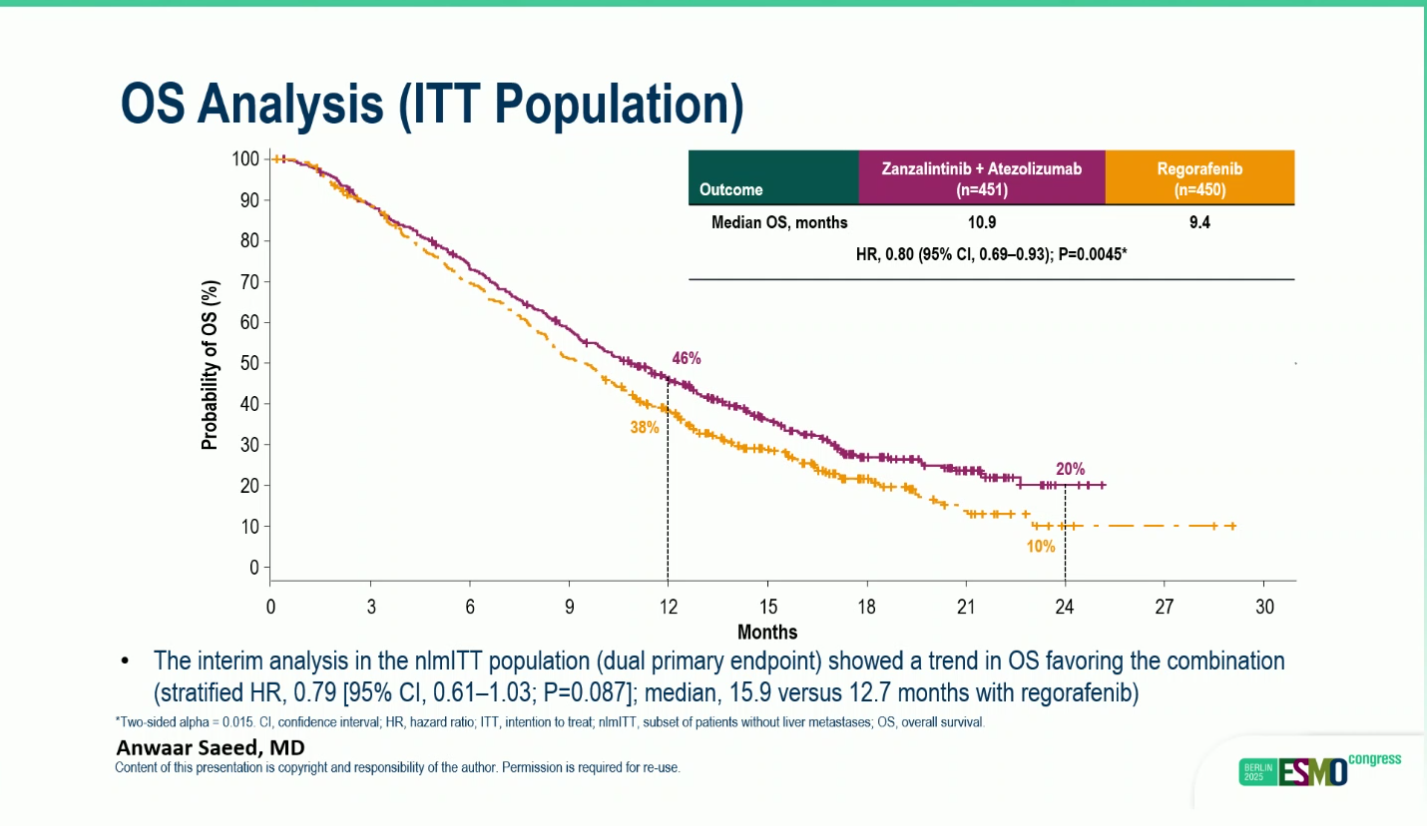
The interim analysis in the nlmITT population (dual primary endpoint) showed a trend toward improved overall survival favoring the zanzalintinib plus atezolizumab combination (stratified HR 0.79; 95% CI, 0.61–1.03; P = 0.087), with a median OS of 15.9 months versus 12.7 months with regorafenib.
- Median PFS: 3.7 vs 2.0 months
- HR: 0.68 (95% CI, 0.59–0.79)
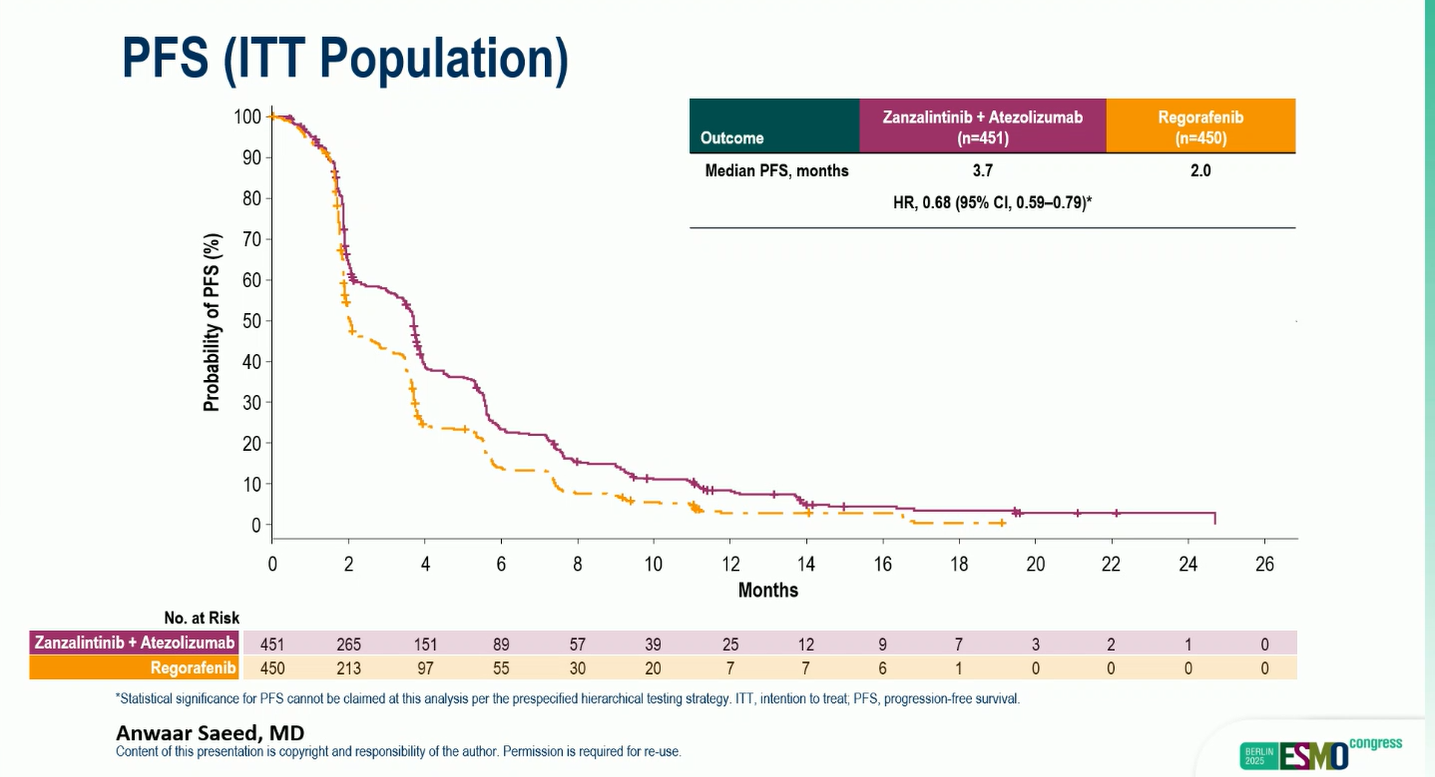
The OS and PFS benefits were consistent across prespecified subgroups, including geographic region, RAS mutation status, presence or absence of liver metastases, and prior anti-VEGF therapy exposure.
Safety Profile
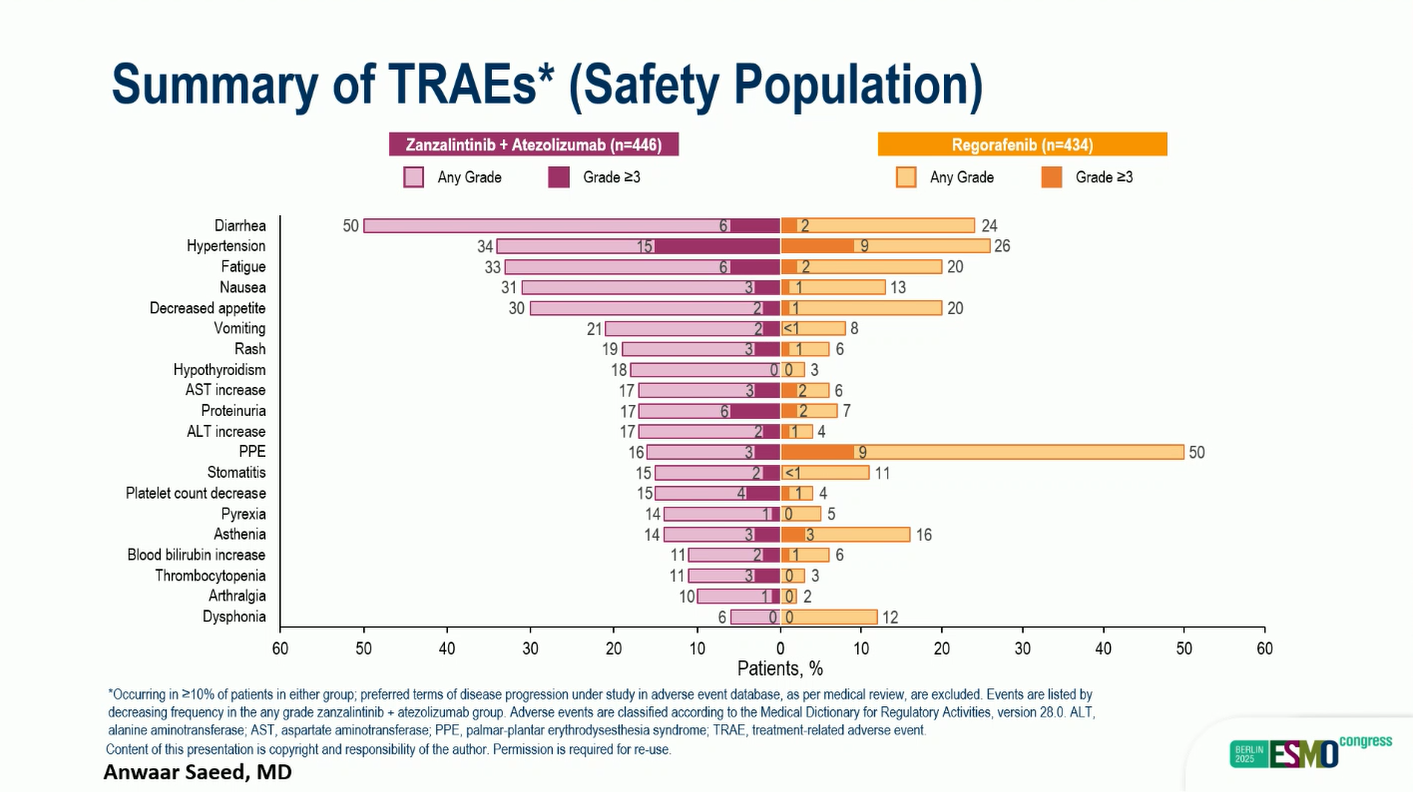
The safety profile of zanza + atezo was manageable and consistent with prior reports. ANy Grade treatment-related adverse events (TRAEs) occurred in 95% of patients receiving zanza + atezo and 92% of those on regorafenib. The most frequent grade 3/4 TRAEs were:
- Hypertension (15% vs 9%)
- Fatigue (6% vs 2%)
- Diarrhea (6% vs 2%)
- Proteinuria (6% vs 2%)
- No new safety signals were observed.
Conclusion
The STELLAR-303 trial, presented by Dr. Anwaar Saeed at the ESMO Congress 2025, marks a major milestone in colorectal cancer research as the first Phase 3 study to demonstrate improved overall survival (OS) with an immune checkpoint inhibitor (ICI)-based combination in metastatic colorectal cancer (mCRC) that is not microsatellite instability-high (MSI-H) or mismatch repair-deficient (dMMR). The trial showed that zanzalintinib plus atezolizumab significantly prolonged OS compared with regorafenib in previously treated MSS mCRC, with the OS benefit consistent across key subgroups—including liver involvement, RAS mutation status, geographic region, and prior anti-VEGF therapy.
Importantly, this combination represents a novel chemotherapy-free regimen capable of producing a meaningful clinical benefit in a population historically refractory to immunotherapy.
You can read the full abstract here.


