iNKT cell therapy is emerging as a critical, next-generation platform designed to overcome the significant hurdles of current immunotherapies. For decades, the field has been dominated by two titans: checkpoint inhibitors (like anti-PD-1) and autologous CAR T-cell therapies. While revolutionary for blood cancers, CAR T-cell therapy has largely struggled with solid tumors and is plagued by significant logistical hurdles.
The 2025 Society for Immunotherapy of Cancer (SITC) Annual Meeting has solidified this shift toward “off-the-shelf,” immune-restorative platforms. Leading this charge is agenT-797, an allogeneic, native invariant natural killer T (iNKT) cell therapy. Updated Phase 1 findings (NCT05108623) presented at SITC 2025 (Late Breaking Abstract #1344) demonstrate that agenT-797 is not just another cell therapy; it is a potent immune-orchestrating agent capable of “re-sensitizing” cold, treatment-resistant tumors and delivering durable, long-term survival.

“Off-the-shelf,” native iNKT cell therapy agenT-797’s Mechanism of Action
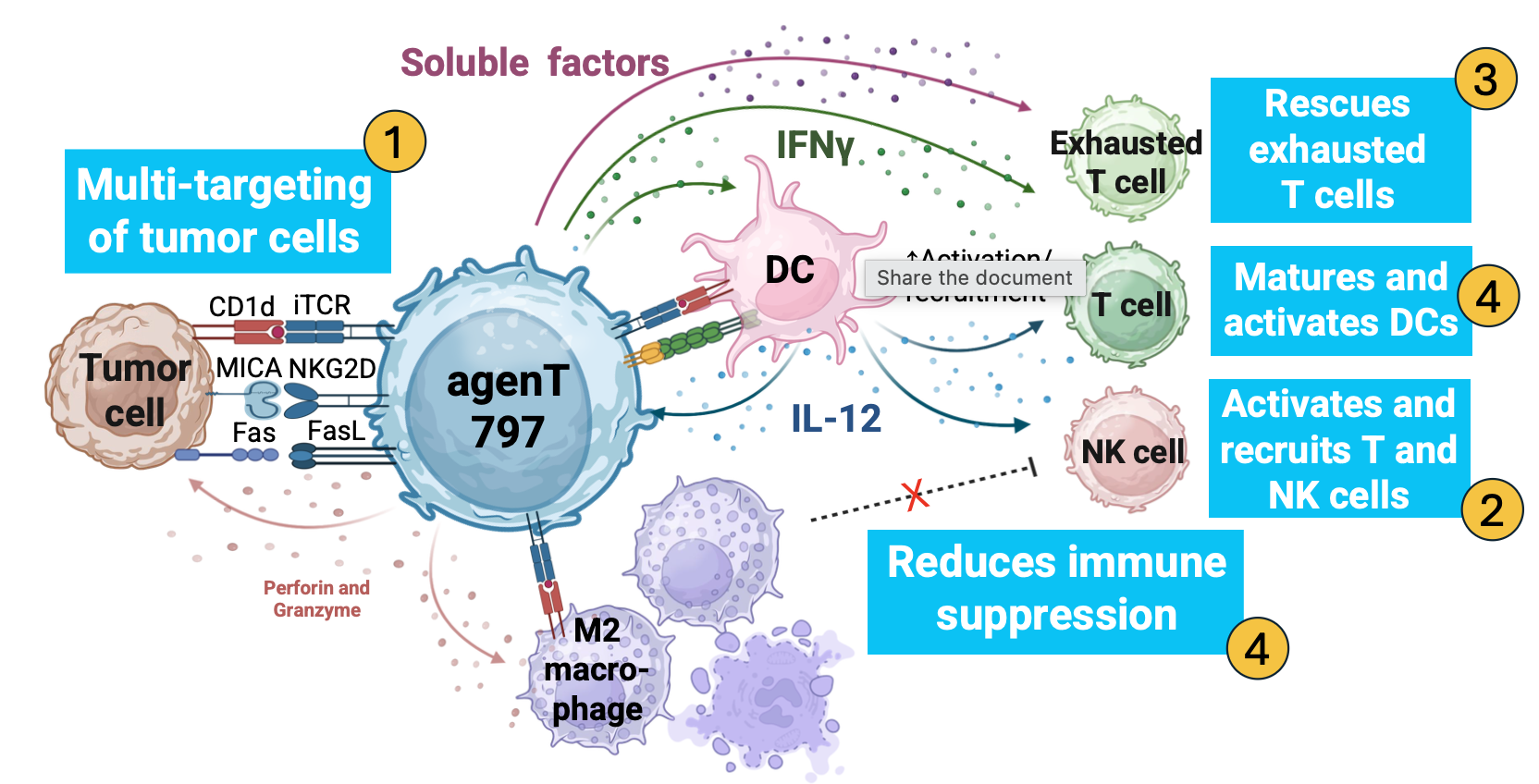
Unlike therapies that perform a single function, agenT-797 is an allogeneic, or “off-the-shelf,” native iNKT cell therapy that acts as a multi-pronged immune modulator. Because iNKT cells do not cause Graft-versus-Host-Disease (GvHD), agenT-797 can be manufactured from healthy donors and administered to patients without any need for HLA matching or harsh lymphodepletion chemotherapy.Its mechanism of action, as detailed in new data presented at the 2025 Society for Immunotherapy of Cancer (SITC), is twofold:
Direct Tumor Killing
The iNKT cells directly target and kill cancer cells through multiple pathways, including both TCR-dependent (via CD1d) and TCR-independent (e.g., NKG2D/MICA) mechanisms.
Source: Poster Presentation at SITC 2025
Immune Re-orchestration
This is the therapy’s key innovation. agenT-797 doesn’t just fight; it “reconstitutes immunity” by fundamentally remodeling the hostile tumor microenvironment (TME) It:
- Rescues Exhausted T-cells: It “rejuvenates” the patient’s own exhausted anti-tumor T-cells, restoring their ability to fight cancer/
- Activates the “Generals”: It matures and activates dendritic cells (DCs), the cells responsible for presenting antigens and coordinating the adaptive immune response.
- Calls in Reinforcements: It actively recruits fresh T-cells and NK cells into the tumor.
- Flips the “Suppressor” Switch: It reprograms immune-suppressive M2 macrophages (which help cancer grow) into pro-inflammatory M1 macrophages (which help kill cancer).
As Dr. Jennifer Buell, President and CEO of MiNK Therapeutics, stated, iNKT cells represent a “new class of immune-restorative therapy” that can act as “master regulators of immune orchestration.”
“AgenT-797 continues to deliver what checkpoint inhibitors alone cannot — durable responses in resistant disease. With a clean safety profile and reproducible activity across tumor types, agenT-797 is well positioned to move into Phase 2 studies and to redefine how we approach immune-resistant cancers.”

Jennifer Buell/LinkedIn
Key Data: Durable Remissions in PD-1-Refractory Cancers
New Phase 1 data (NCT05108623) presented at the SITC 2025 Annual Meeting highlights the clinical potential of agenT-797, an allogeneic, “off-the-shelf” iNKT cell therapy. In heavily pre-treated patients with PD-1 refractory solid tumors, agenT-797 combined with anti-PD-1 therapy demonstrated a median Overall Survival (mOS) of 23.0 months, a significant extension compared to 5.6 months for monotherapy. Mechanistic data confirmed agenT-797 drives immune re-orchestration by activating dendritic cells and rescuing exhausted T-cells, all while maintaining a favorable safety profile with no DLTs, Grade 3 CRS, or neurotoxicity reported
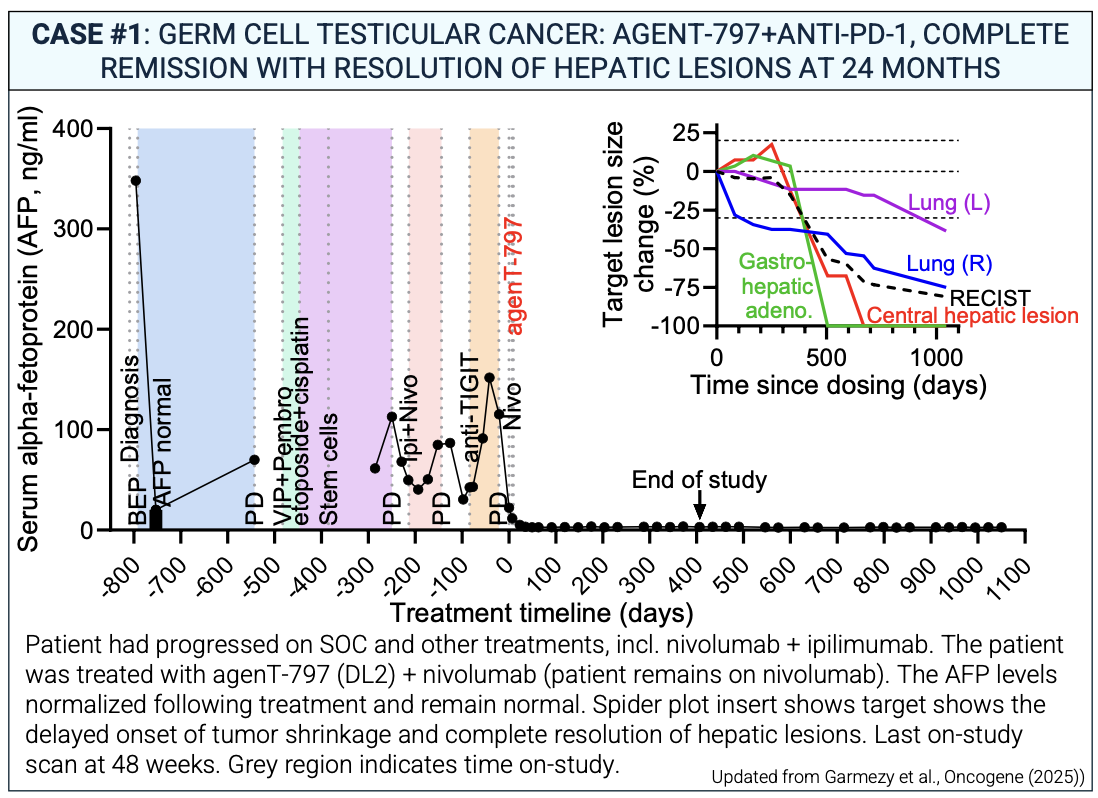
Case #1: Complete Remission in Testicular Cancer
A patient with metastatic germ cell testicular cancer, who had progressed on multiple prior therapies, was treated with agenT-797 plus anti-PD-112. The patient achieved a complete clinical, radiologic, and biochemical remission13. The hepatic lesions resolved, and tumor markers (AFP) normalized14. As of the data cutoff, this complete remission has been sustained for more than 24 months.
Case #2: Durable Response in Gastric Cancer
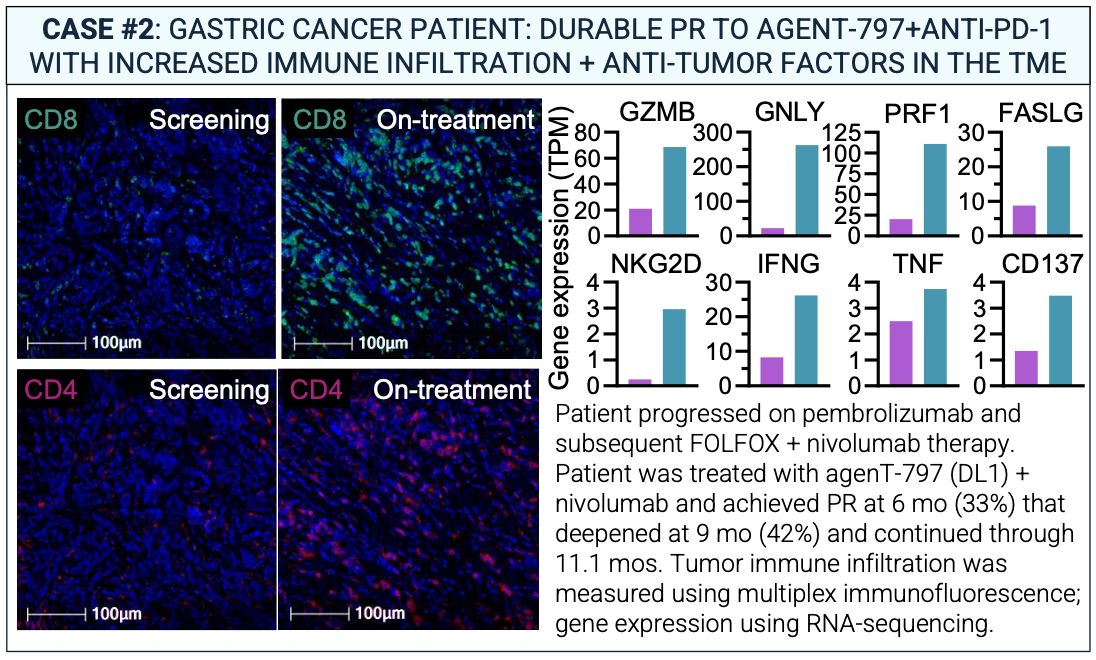
A patient with 2L gastric cancer, who had also progressed on prior treatment, received agenT-797 plus anti-PD-116. This patient achieved a durable partial response (PR)17. Biopsies confirmed the mechanism, showing that post-treatment, the tumor was newly infiltrated with a wave of CD8+ T-cells and other anti-tumor factors (GZMB, PRF1, FASLG).
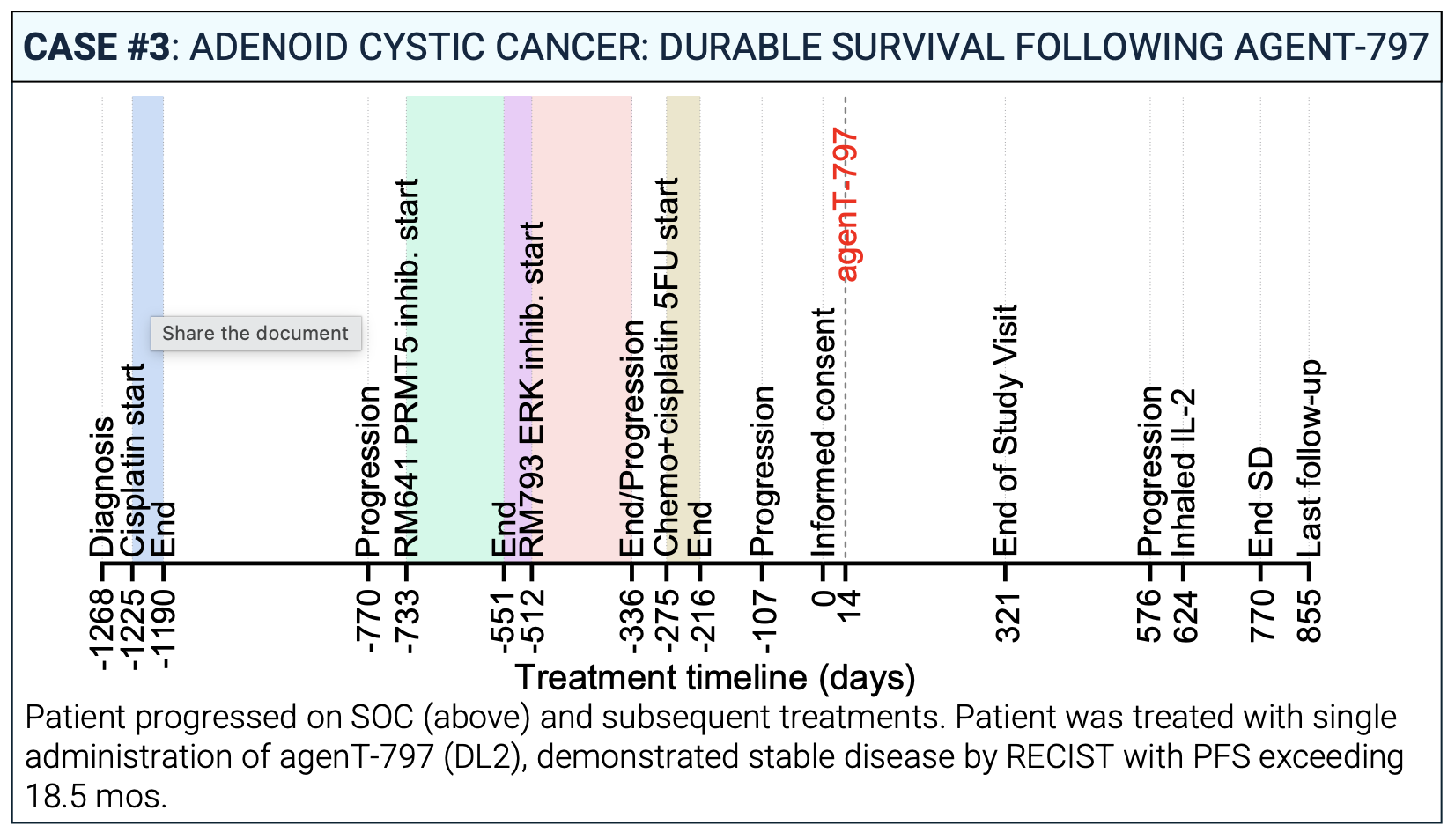
Case #3: Monotherapy Activity in Adenoid Cystic Carcinoma
Even without a checkpoint inhibitor, agenT-797 showed durable activity. A patient with adenoid cystic carcinoma treated with agenT-797 monotherapy demonstrated stable disease with a progression-free survival (PFS) exceeding 18.5 months. Durable survival and disease stabilization were also seen across a wide range of other “cold” tumors, including thymoma, cholangiocarcinoma, and renal cancer, underscoring the therapy’s broad potential.
Safety profile
The Safety Profile of AgenT-797 was reported to be favourable, across all tumor types. Across all 34 patients treated, agenT-797 was exceptionally well-tolerated:
- No Dose-Limiting Toxicities (DLTs) were observed.
- No Grade 3 or higher CRS was seen.
- No neurotoxicity (ICANS) of any grade was reported.
The most common treatment-related adverse event was fatigue (n=7). Only one patient experienced Grade 1 CRS (2.9% of all patients). This “clean safety profile” not only makes it a much safer option for patients but also strongly supports its use in combination regimens and for repeat dosing.
Understanding iNKT Cell Therapy: The Next-Generation “Off-the-Shelf” Solution
iNKT cell therapy utilizes invariant Natural Killer T cells, a rare, innate-like T-cell population that skillfully bridges the gap between innate and adaptive immunity. Unlike conventional T cells, iNKT cells recognize lipid antigens presented by CD1d molecules, a unique mechanism that allows them to target a broader range of tumors, including those with low MHC expression often resistant to standard therapies. Critically, agenT-797 is an allogeneic (universal donor) native iNKT cell therapy, enabling it to be mass-produced, cryopreserved, and delivered rapidly as an off-the-shelf product, eliminating the manufacturing and logistical nightmares associated with autologous therapies.
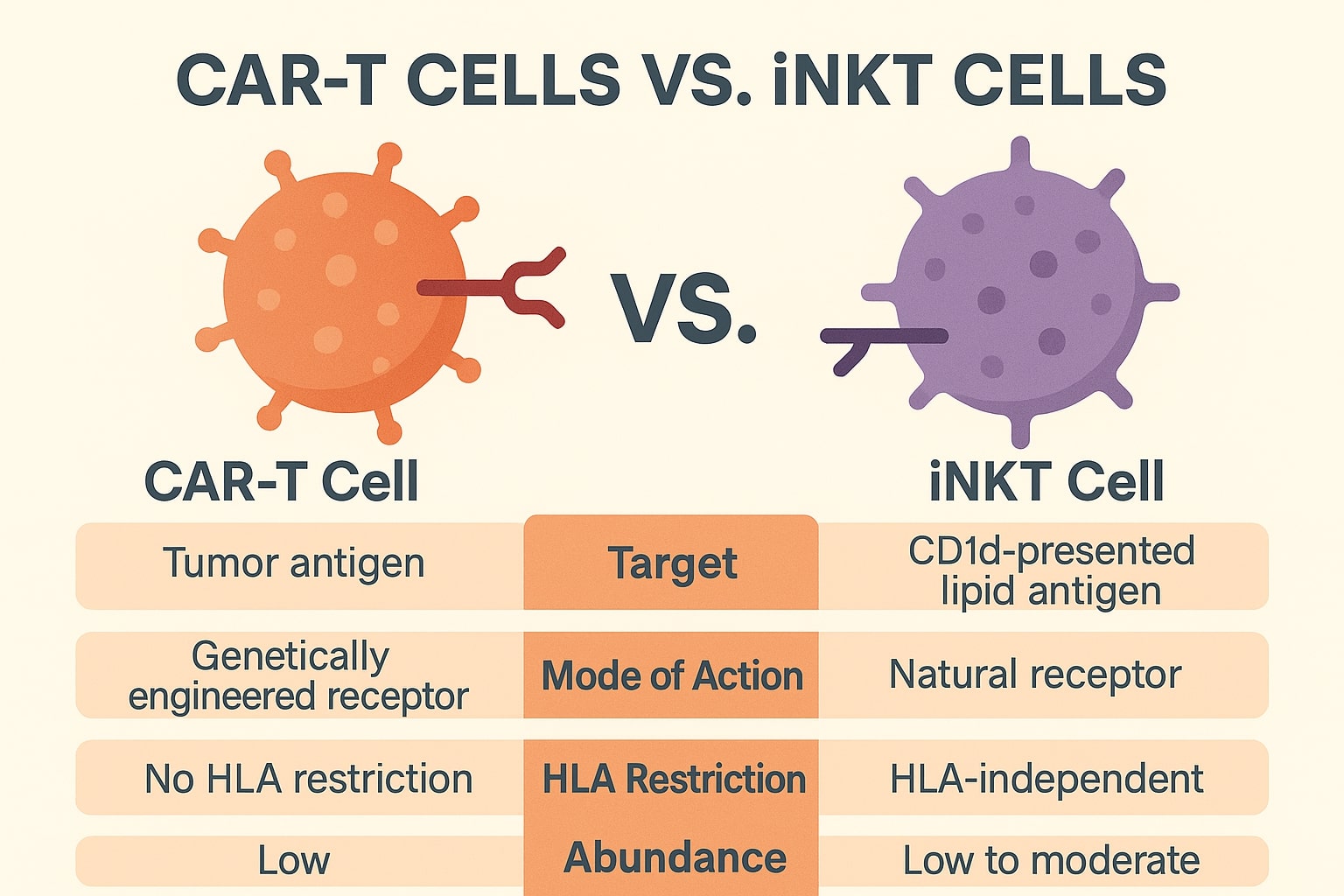
The fundamental difference in the CAR T-cell therapy vs iNKT cell therapy debate lies in accessibility and safety. CAR T-cells are personalized, autologous products tailored to each patient, which results in high costs, 3-4 week “vein-to-vein” processing times, and a frequent risk of severe toxicities like Grade 3 Cytokine Release Syndrome (CRS) and neurotoxicity (ICANS). In sharp contrast, iNKT cell therapies are inherently resistant to Graft-versus-Host Disease (GvHD) and demonstrate a significantly safer toxicity profile, with minimal CRS and negligible ICANS, making them uniquely scalable and suitable for broader use, especially in the challenging environment of solid tumors where CAR T-cells have largely struggled.
Who Manufactures agenT-797?
AgenT-797 is manufactured and developed by MiNK Therapeutics, Inc., a clinical-stage biopharmaceutical company focused on pioneering allogeneic invariant natural killer T (allo-iNKT) cell therapies. MiNK Therapeutics is actively leveraging its proprietary, US-based manufacturing and platform infrastructure to advance its pipeline, which includes Phase 2 trials for agenT-797 in gastric cancer and severe pulmonary disease, and a Phase 2 study in germ-cell cancer. The company positions itself as a leader in the development of iNKT cell therapy to reconstitute immunity for treating cancer and immune disorders.
The Takeaway: A New Pillar of Immunotherapy
The data on agenT-797 provides a clear, compelling case for iNKT cells as a new pillar of cancer treatment. It validates the platform as an “off-the-shelf,” immune-restorative therapy that can deliver what checkpoint inhibitors and CAR T-cells alone cannot: durable responses in immune-resistant solid tumors.
“We’re seeing encouraging clinical activity with agenT-797 — including durable responses and deep remissions that have persisted beyond two years in some patients,”
said Dr. Ben Garmezy, Associate Director of Genitourinary Research at Sarah Cannon Research Institute and presenting author at SITC.

By acting as a “master regulator,” agenT-797 does more than just kill cancer cells—it teaches the patient’s own immune system how to recognize and fight the disease again. With a 23-month median survival in PD-1-refractory patients and a pristine safety profile, agenT-797 is not just a promising new drug; it may be the key to unlocking the full potential of immunotherapy for all.
Read More About iNKT vs Car-T Cell Therapies on OncoDaily