On September 10, 2025, Revolution Medicines announced new clinical data for daraxonrasib (RMC-6236), its investigational RAS(ON) inhibitor, showing durable benefit in previously treated patients and highly encouraging activity in the first-line setting for metastatic pancreatic ductal adenocarcinoma (PDAC). These results provide strong support for the launch of RASolute 303, a global Phase 3 registrational trial expected to begin enrollment later this year.
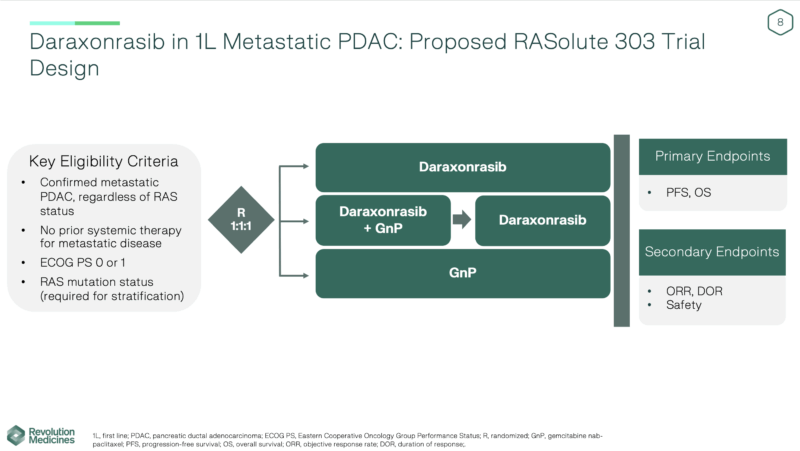
RASolute 303 Trial Design
The global, randomized Phase 3 RASolute 303 trial will enroll patients with newly diagnosed metastatic PDAC, regardless of RAS mutation status, provided they have no prior systemic therapy for metastatic disease and an ECOG performance status of 0 or 1.
Participants will be randomized equally (1:1:1) into three arms:
- Daraxonrasib monotherapy (300 mg once daily)
- Daraxonrasib in combination with gemcitabine plus nab-paclitaxel (GnP) (daraxonrasib given at 200 mg QD in this combo)
- Standard-of-care GnP chemotherapy alone
Primary and Secondary Endpoints of RASolute 303 Trial
The primary endpoint is progression-free survival (PFS), overall survival (OS), independently assessed using RECIST v1.1 criteria.
Secondary endpoints include, objective response rate (ORR), duration of response (DoR), disease control rate (DCR), safety and tolerability, and patient-reported quality-of-life outcomes.
Clinical Results
The latest data offer a comprehensive view of daraxonrasib’s performance across multiple treatment settings for metastatic PDAC. Long-term follow-up in the second-line population confirmed durable responses and a consistent safety profile, while early results in the first-line setting showed promising activity both as monotherapy and in combination with standard chemotherapy.
Long-Term Outcomes in Second-Line PDAC
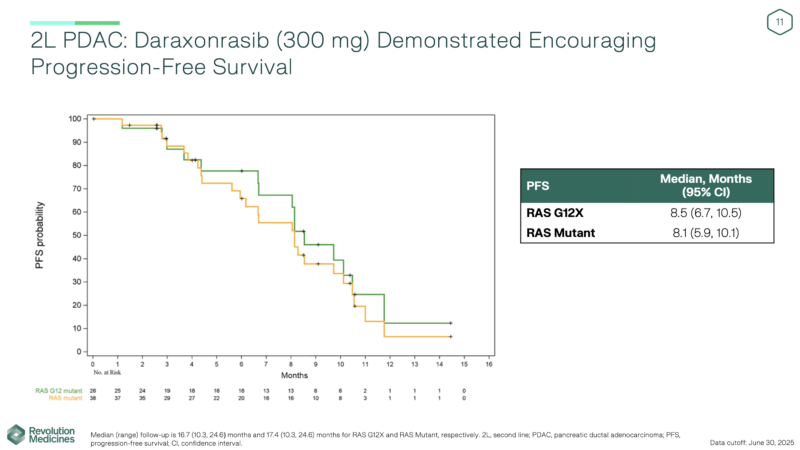
In 83 patients with RAS-mutant 2L+ metastatic PDAC treated with daraxonrasib 300 mg once daily, clinical activity remained compelling:
- ORR: 35% (RAS G12X, n=26) and 29% (all RAS mutations, n=38)
- DCR: 92% and 95%, respectively
- Median DoR: 8.2 months
- Median PFS: 8.5 months (RAS G12X) and 8.1 months (all mutations)
- Median OS: 13.1 and 15.6 months, respectively
- Median follow-up: ~17 months
Safety profile
The safety profile was consistent and manageable. Almost all patients (96%) experienced treatment-related adverse events, most commonly rash, which occurred in 90% of patients, and mucositis or stomatitis, reported in just over half. Severe events (grade 3 or higher) were seen in about one-third of patients. Treatment adjustments were common but effective: 43% required temporary dose interruptions and 30% needed dose reductions, yet no patients discontinued therapy because of toxicity. The mean dose intensity remained high at 86%, highlighting the overall tolerability of the regimen.
Encouraging Data in First-Line PDAC
Initial data from the first-line setting provide a clear look at daraxonrasib’s activity and safety profile, both as monotherapy and in combination with chemotherapy.
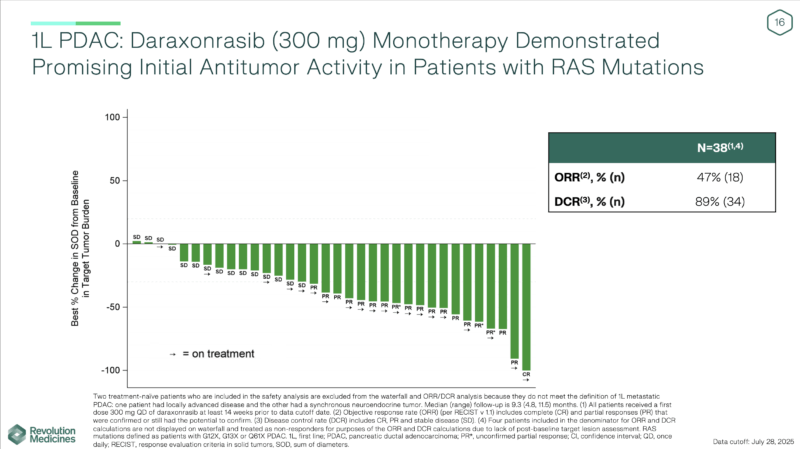
Daraxonrasib Monotherapy
At the July 28, 2025 cutoff, daraxonrasib 300 mg once daily showed encouraging activity in treatment-naïve RAS-mutant metastatic PDAC. The safety profile was manageable and consistent with prior reports, with a mean dose intensity of 85%. Among 38 evaluable patients, the objective response rate (ORR) reached 47% and the disease control rate (DCR) 89%, with a median follow-up of 9.3 months. Most patients remained on treatment at data cutoff, suggesting ongoing benefit.
Daraxonrasib + GnP Combination
The combination of daraxonrasib 200 mg daily with gemcitabine and nab-paclitaxel (GnP) produced even stronger activity. In 31 patients with sufficient follow-up, the ORR was 55% and the DCR 90%, with a median follow-up of 6.9 months. The regimen was well tolerated and maintained an 81% mean dose intensity, with no new safety concerns.
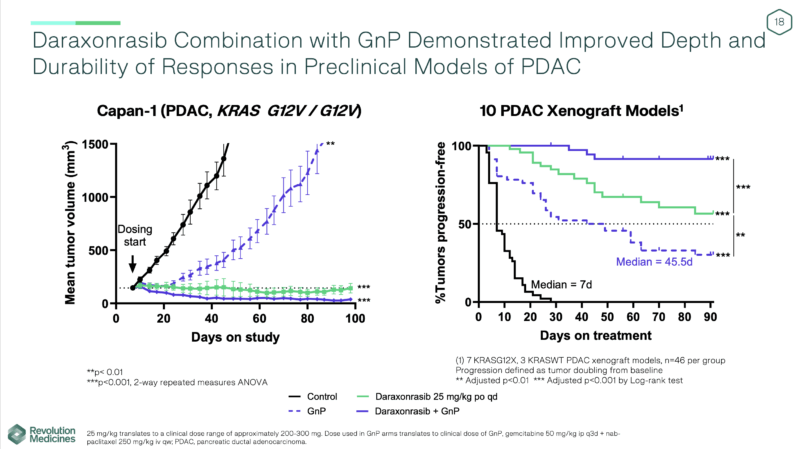
Earlier Trial Results of Daraxonrasib in PDAC
In July 2024, updated data from the Phase 1/1b trial of daraxonrasib (RMC-6236) were reported by Revolution Medicines, Inc., further validating its potential in metastatic PDAC. At the 300 mg once-daily dose — the same regimen used in the ongoing RASolute 302 Phase 3 study — daraxonrasib was generally well tolerated, with rash and mild gastrointestinal events as the most frequent treatment-related adverse events, mostly grade 1–2. No grade ≥3 events occurred in more than 10% of patients, there were no treatment discontinuations due to toxicity, and the mean dose intensity was 89%.
Among 37 patients with second-line RAS-mutant PDAC, clinical activity was compelling. The objective response rate (ORR) was 36% in KRAS G12X tumors and 27% across all RAS mutations. The median progression-free survival (PFS) reached 8.8 months in KRAS G12X and 8.5 months in all RAS mutations, while median overall survival (OS)had not yet been reached. Six-month survival rates were 100% and 97%, respectively, demonstrating durable benefit.
Find more information on Revolution Medicines, Inc. Official Website.
What is Daraxonrasib and How does it work?
Daraxonrasib (RMC-6236) is being developed and produced by Revolution Medicines, Inc., a late-stage clinical oncology company focused on targeted therapies for RAS-addicted cancers. By targeting key mutations (G12X, G13X, Q61X), it broadly suppresses RAS-driven signaling, a hallmark of PDAC biology. More than 90% of PDAC tumors carry RAS mutations, making RAS inhibition one of the most promising strategies for improving outcomes in this difficult-to-treat cancer.
Conclusion
The growing body of evidence for daraxonrasib (RMC-6236) paints a consistent picture: meaningful responses, prolonged disease control, and a safety profile that allows patients to stay on therapy. Long-term results in second-line metastatic PDAC confirm durable benefit, while first-line data — both as monotherapy and in combination with gemcitabine and nab-paclitaxel — show even higher response rates and encourage further evaluation.
With the global Phase 3 RASolute 303 trial set to begin enrollment later this year, daraxonrasib is now positioned to potentially redefine the standard of care for patients with RAS-mutant metastatic PDAC. The ongoing Phase 3 RASolute 302 trial evaluating daraxonrasib monotherapy in second-line metastatic PDAC is expected to finish global enrollment this year, with data readout anticipated in 2026.