The ESMO 2025 Congress is a major global oncology event organized by the European Society for Medical Oncology (ESMO).
It is taking place at Messe Berlin in Berlin, Germany, from October 17 to 21, 2025. The congress features a comprehensive scientific and educational program designed to foster exchange and debate in translational cancer science, showcasing potentially practice-changing data, and stimulating multidisciplinary discussions to improve cancer treatment options.
“Excellent talk on somatic and germline testing of advanced prostate cancer by Channing Paller.
All these pts should get tested. Guidelines by different countries.”

“Today my friend and colleague Luca Arecco presented very interesting data on Brain mts at ESMO25 – a very relevant topic in cancer care. Make sure you don’t miss his great presentation.”

“Great PROs presentation from ASCENT-04 by Evandro de Azambuja at ESMO25.
ASCENT-04, SG+pembro maintained QoL vs chemo+pembro in PD-L1+ mTNBC, with improved emotional function (HR 0.71), less pain (0.75) and insomnia (0.75), consistent safety profile.”

“QoL improvement is an important goal in advanced cancer.
In SERENA 6, early shift to camizestrant demonstrated consistent benefit in delayed time to deterioration of PRO cancer symptoms (pain, fatigue, dyspnoea) and functioning.”

“Talking about quality of life without including the patient’s perspective is not really what I would expect in 2025 at a conference like ESMO25.”
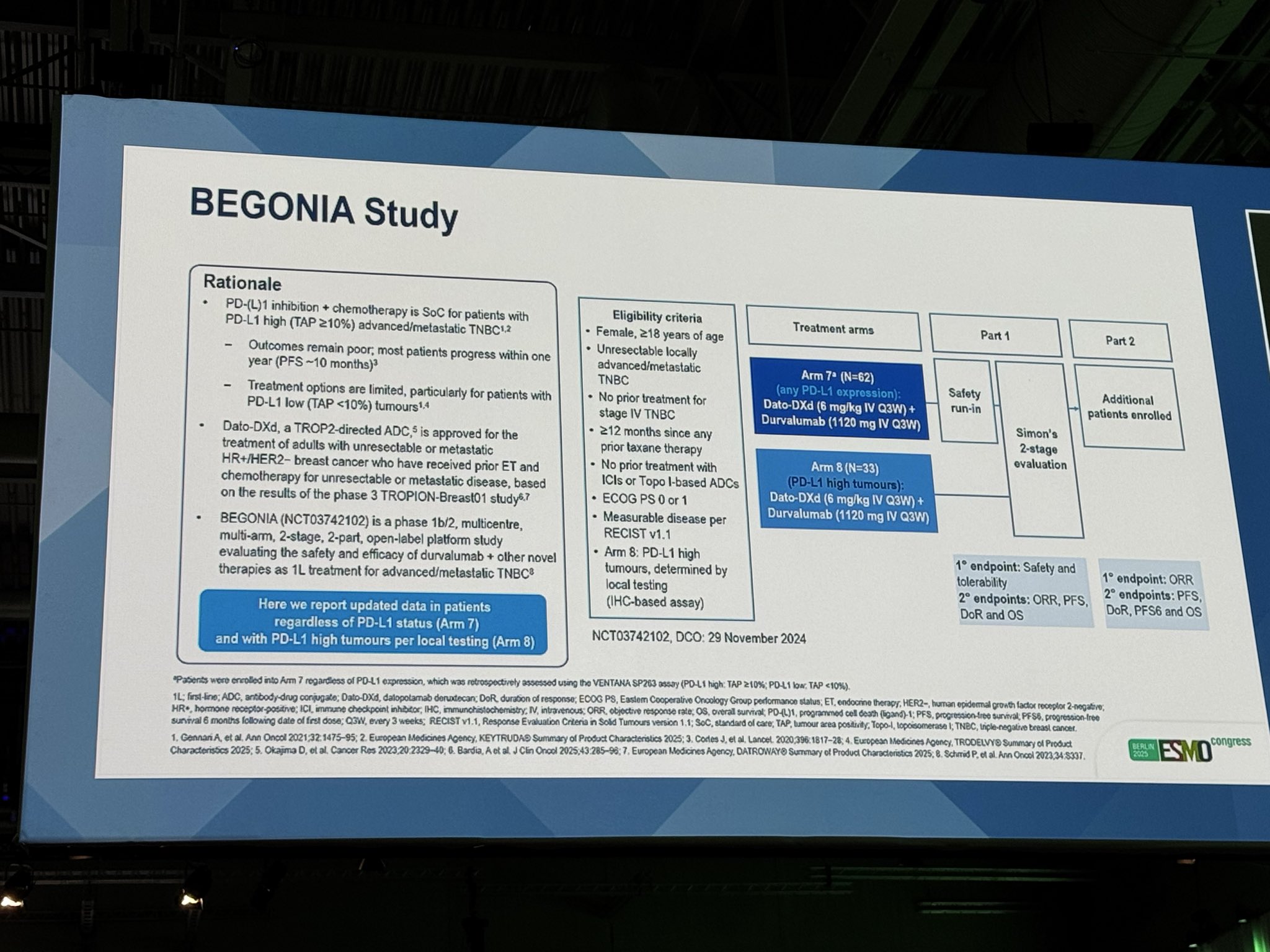
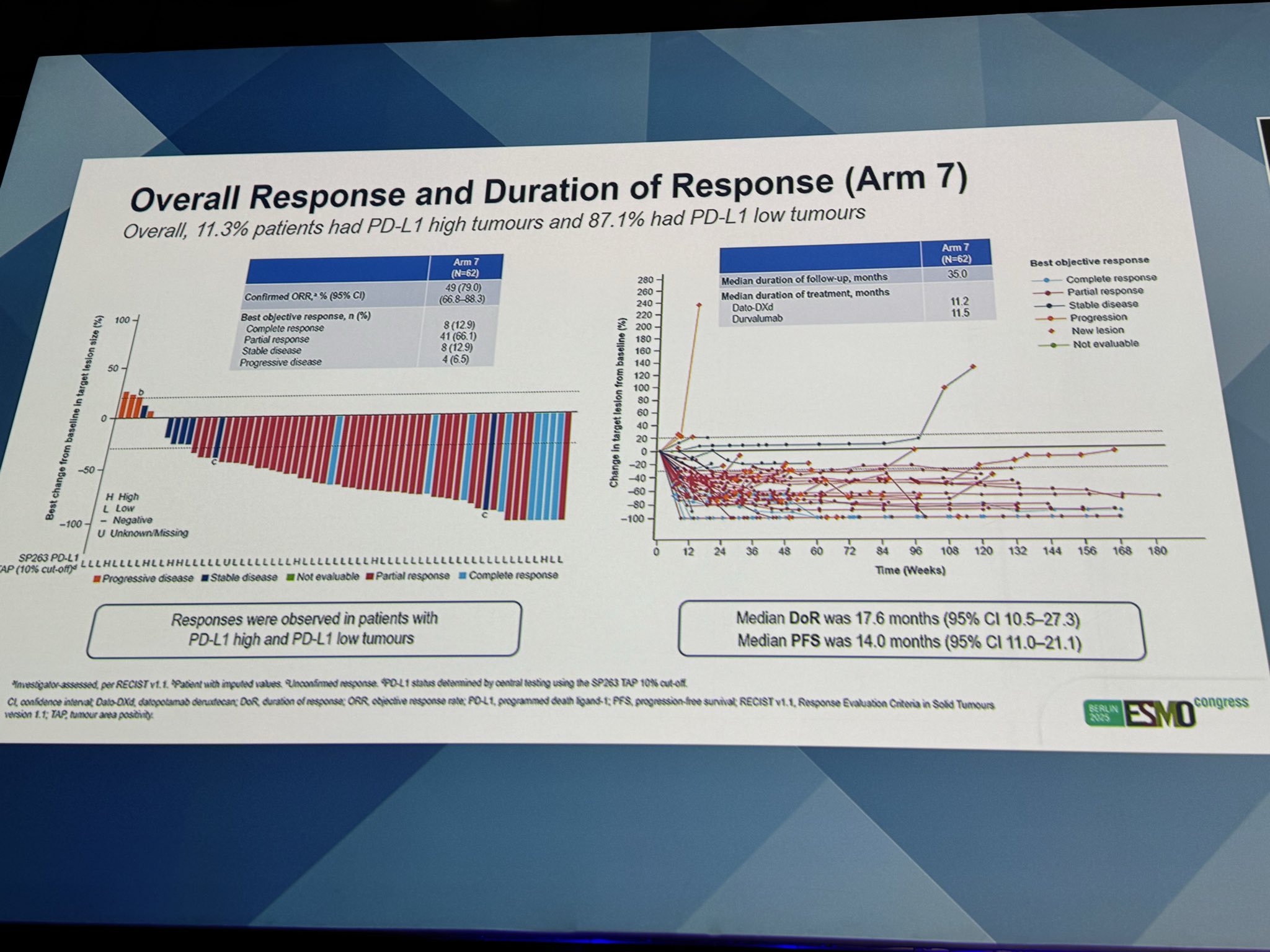
“BEGONIA: Dato-DXd + durvalumab in mTNBC
ARM 7 ,PDL1-: ORR 79% , DOR 17.6 mo, (35 mo f/u), PFS 14mo
Arm 8 PDL1+: ORR 81.8% (10.7 mo f/u), PFS immature.”
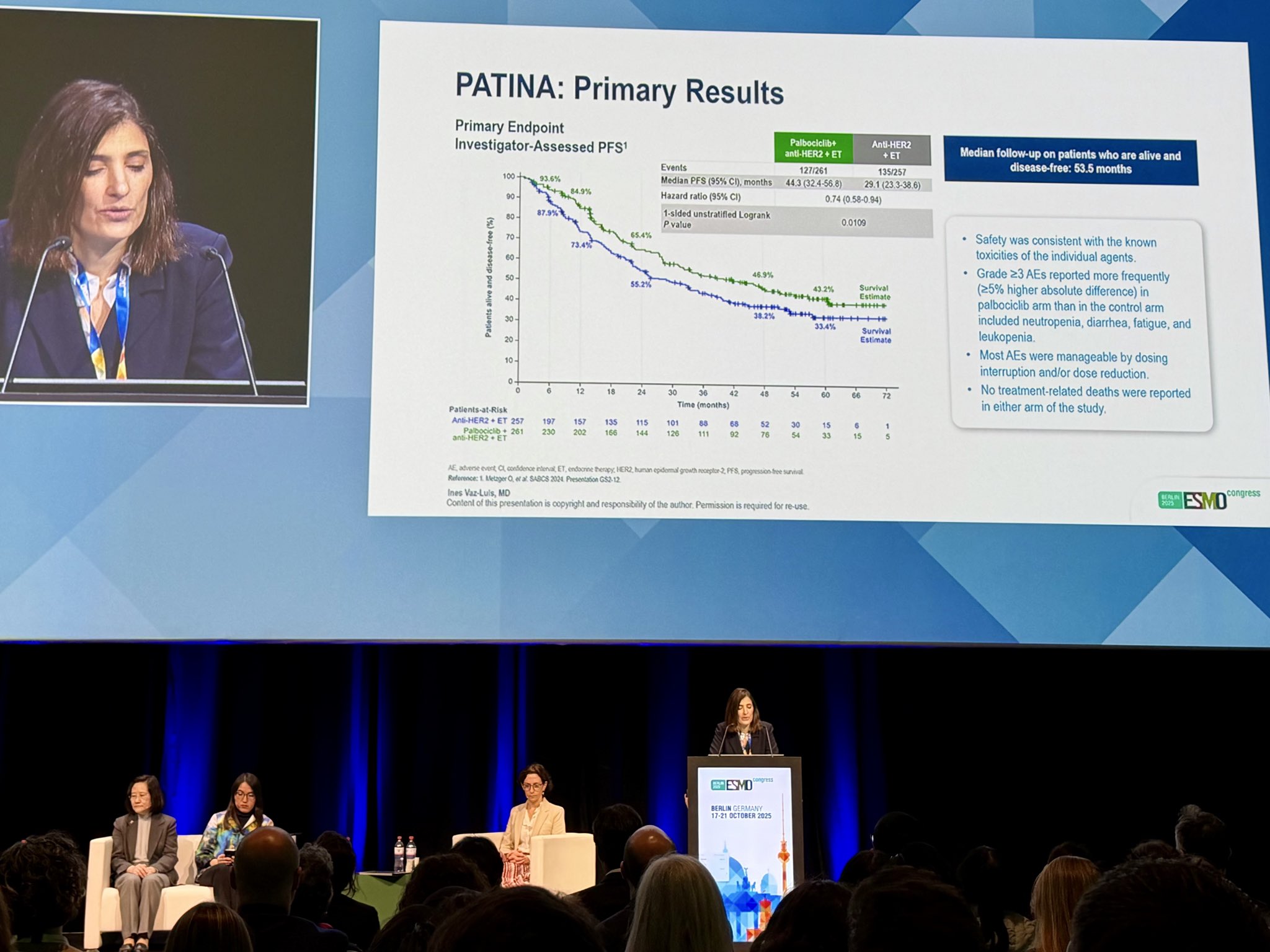
“Excellent presentation by Ines Vaz Luis:
HRQoL results from the practice-changing PATINA (AFT-38) trial show that adding palbociclib to HER2 therapy + ET preserves quality of life over long-term treatment, supporting its role in HR+/HER2+ MBC as 1st line treatment.”
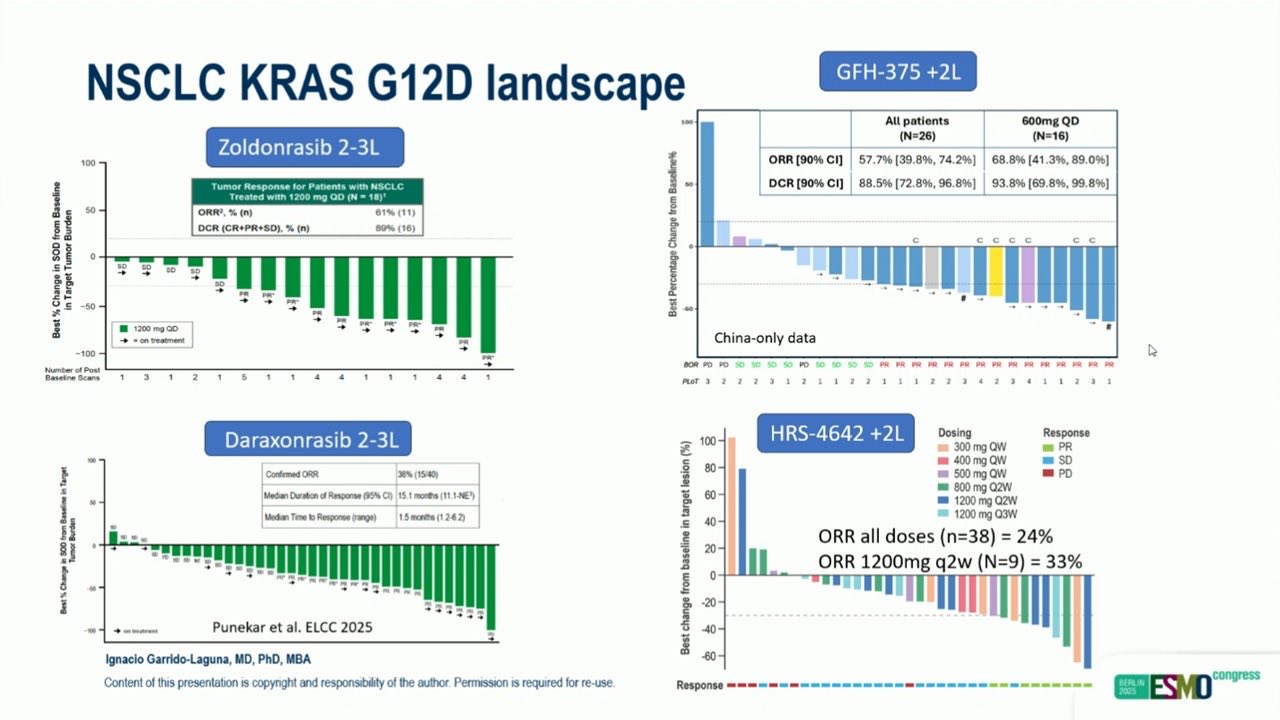
“KRAS inhibitors in NSCLC:
- G12Di:coming soon to meet a major unmet need – same activity in smokers vs never-smokers?
- G12Ci: expanding field, active in both naïve and post-sotorasib/adagrasib patients.
Excellent summary Ignacio Garrido-Laguna.”
“Your myESMOStory moments made ESMO’s 50th anniversary special.
From selfies at ESMO25 to memories shared from afar, together we marked 50 years of progress in oncology.
Here’s to accelerating impact!”

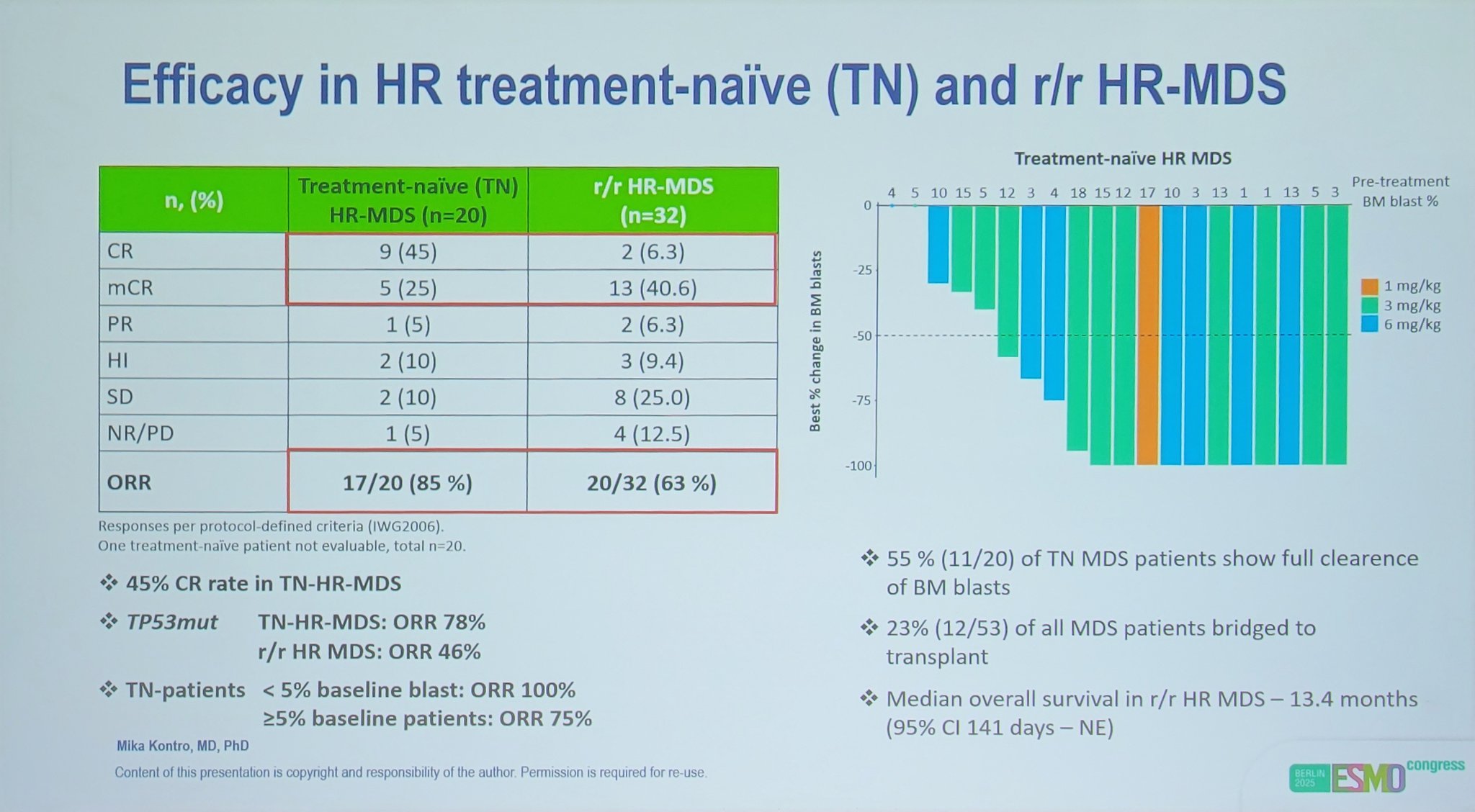
“Data presented at ESMO25 by Dr Mika Kontro on the Macrophage reprogrammer bexmarilimab plus azacitidine in myelodysplastic syndrome (BEXMAB).”
” BEGONIA study ESMO25 Amazing results for Dato-DXd + durvalumab as 1L treatment for advanced/metastatic TNBC!
Arm 7 (all PD-L1 levels)
- ORR 79.0%
- mDoR 17.6 mo, mPFS 14.0 mo
- Durable responses regardless of PD-L1 status
Arm 8 (PD-L1 high)
- ORR 81.8%
- Manageable safety, no new safety signals Dato-DXd + durvalumab demonstrated robust and consistent antitumor activity!”
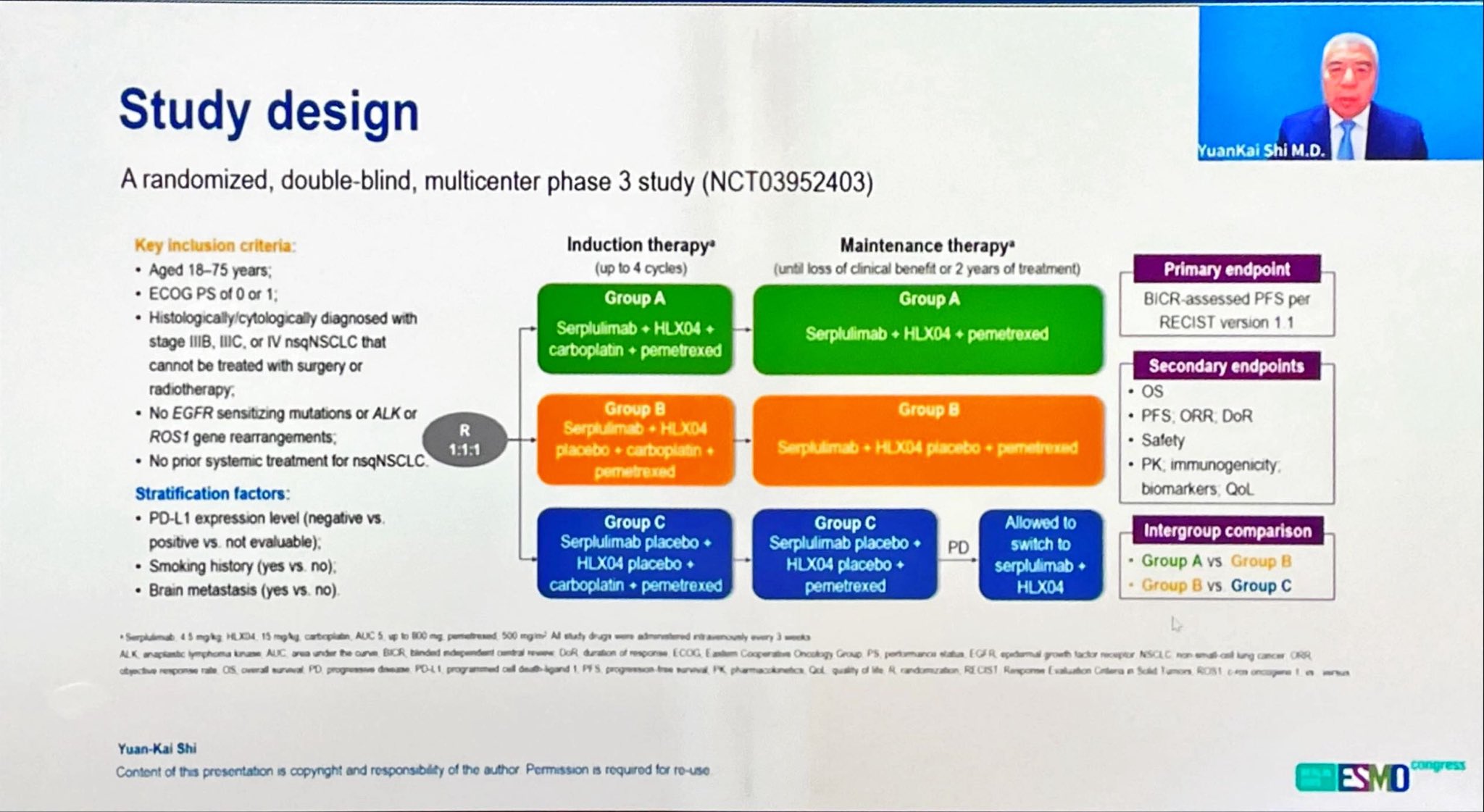
“ASTRUM-002 phase Ill study Serplulimab plus chemotherapy with or without HLXO4 (bevacizumab biosimilar) in advanced nonsquamous NSCLC.
While serplulimab improved both PFS and OS, bevacizumab biosimilar did not have additive efficacy with serplulimab. Control arm based on chemo only is no longer the standard of care.”
“At ESMO25 Luca Arecco from Jules Bordet Institute, presents results from the Brainstorm study.
CSF ctDNA in pts with brain and LM metastases had high mutational profile concordance with plasma and only partial overlap with extra-CNS tissue, while also uncovering CNS-specific mut.”

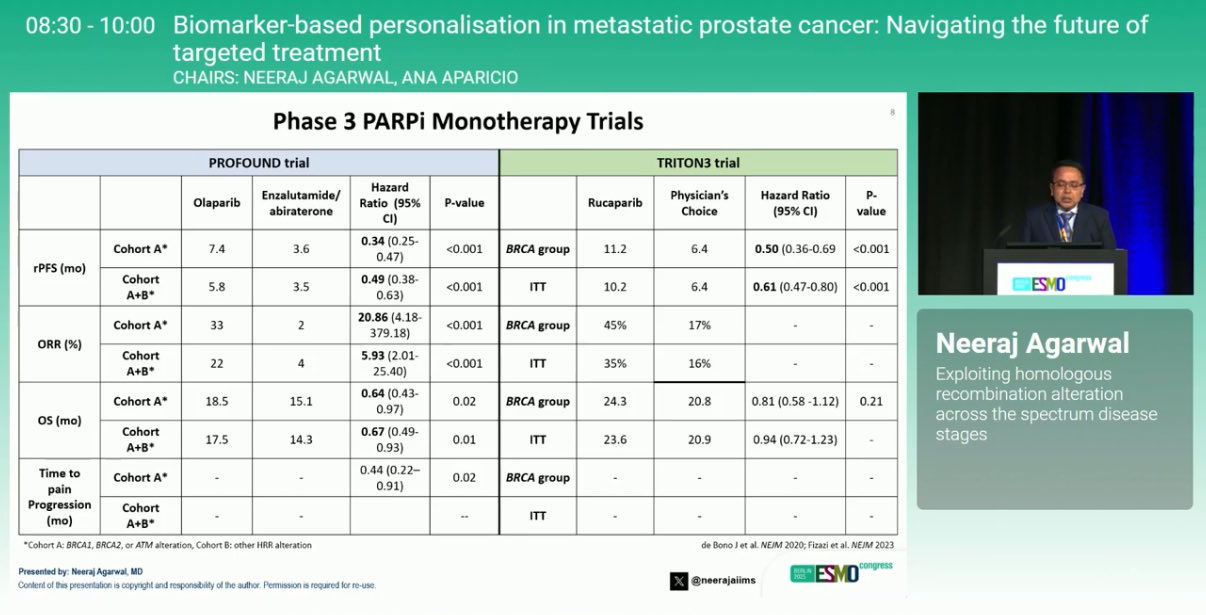
“Thorough, insightful review of PARP inhibitors in prostate cancer from Neeraj Agarwalm– monotherapy and rationale for combos.
Important to test our patients for HRR alterations!”
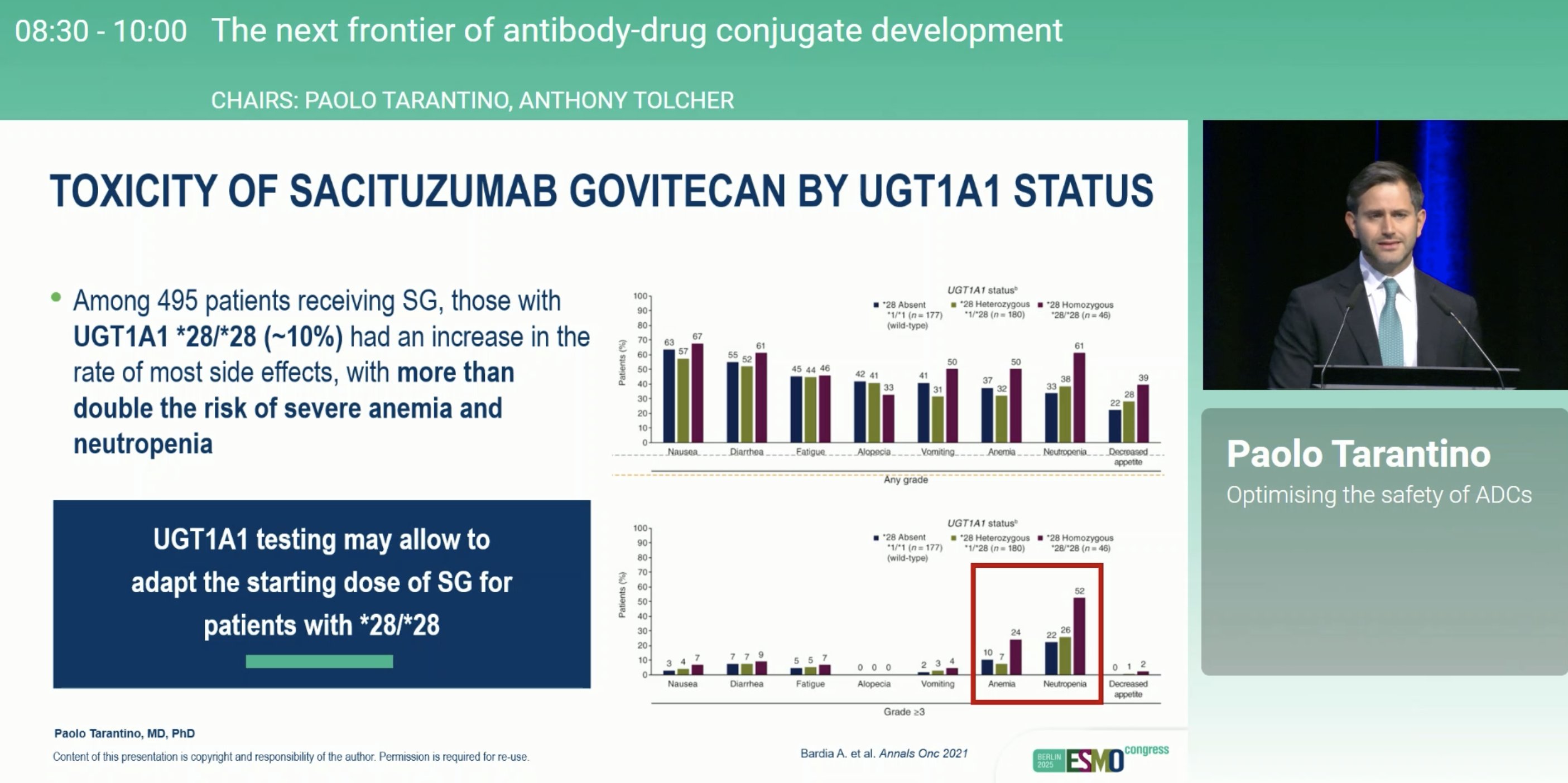
“Insightful presentation by Paolo Tarantino at ESMO25 on ADC toxicity strategies in drug development.
We need to take learnings from decades of cytotoxic chemotherapy to ensure that ADCs are safe+tolerable. Our IBI343 CLDN18.2 ADC e.g. Fc silenced ADC.”
“Prof. Schmid presents BEGONIA: dato-DXd + durva in mTNBC.
PDL-1 neg (arm 7) ORR 79%, PFS 14 mo.
PDL-1 pos (arm 8) ORR 81.8%, PFS immature (good sign they were on longer).
Safety doesn’t look much different.”
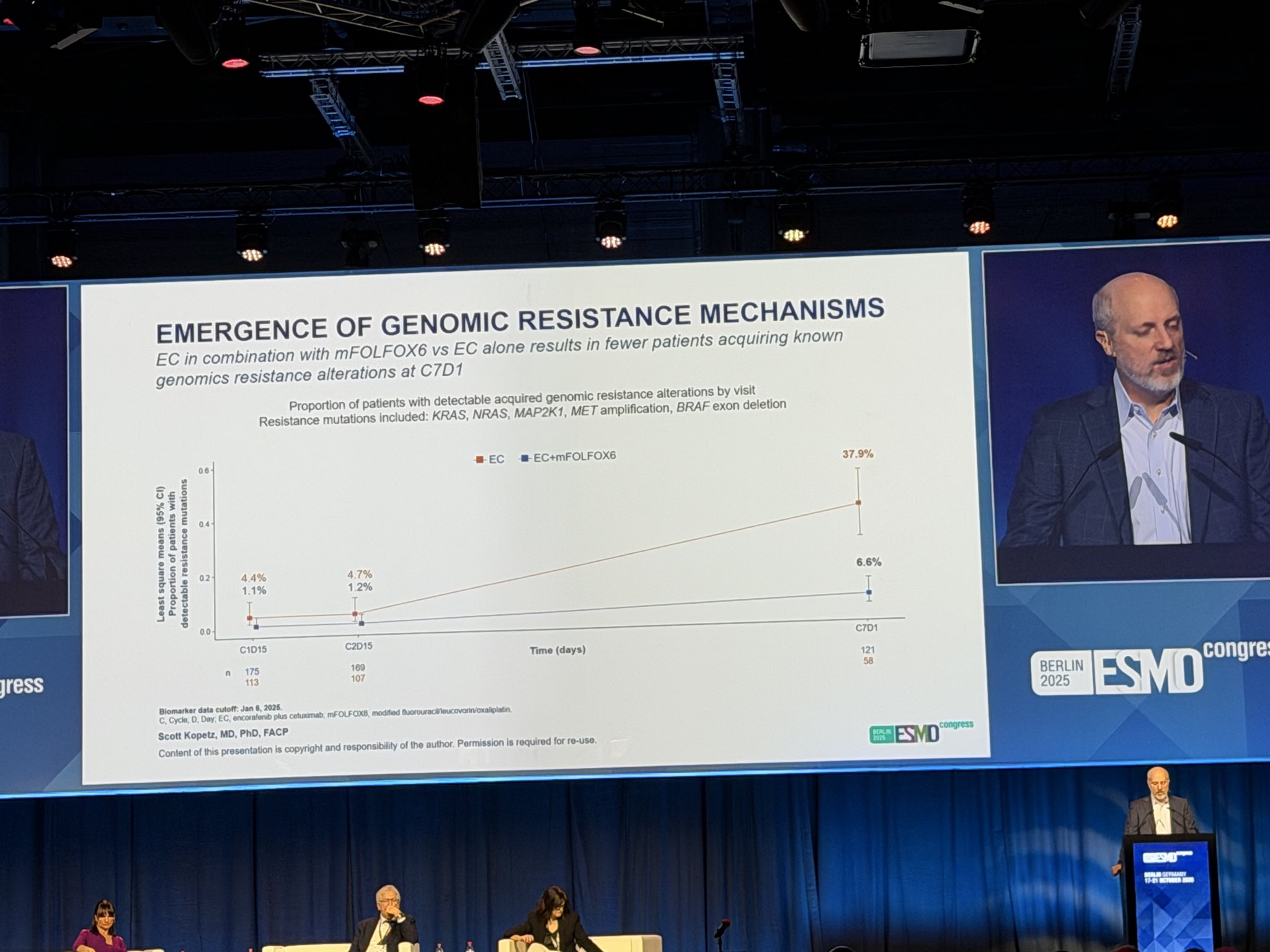
“Does chemotherapy affect the biology of BRAF V600E mCRC when combined with encorafenib + ceruximab?
Answer appears to be YES! Very intriguing translational finding showing that addition of chemo to E+C reduces development of acquired RAS, MAP2K1 , and other mutations that drive resistance to BRAF + EGFR blockade.
Will use this data in counseling patients why we add chemo to E+C in this pt population. to BREAKWATER team for releasing translational data on their large 500 pt ( ) analysis!! And congrats to the always amazing Scott Kopetz for an incredible presentation- our MD Anderson Cancer Center team remains so inspired by you!!”
“Even within the same country (Italy), use of off-label cancer drugs is subject to significant inter-hospital variability. This leads to potential disparities in patients’ access to care.
Have a look at the project by AIOM, just published on Tumori Journal.”
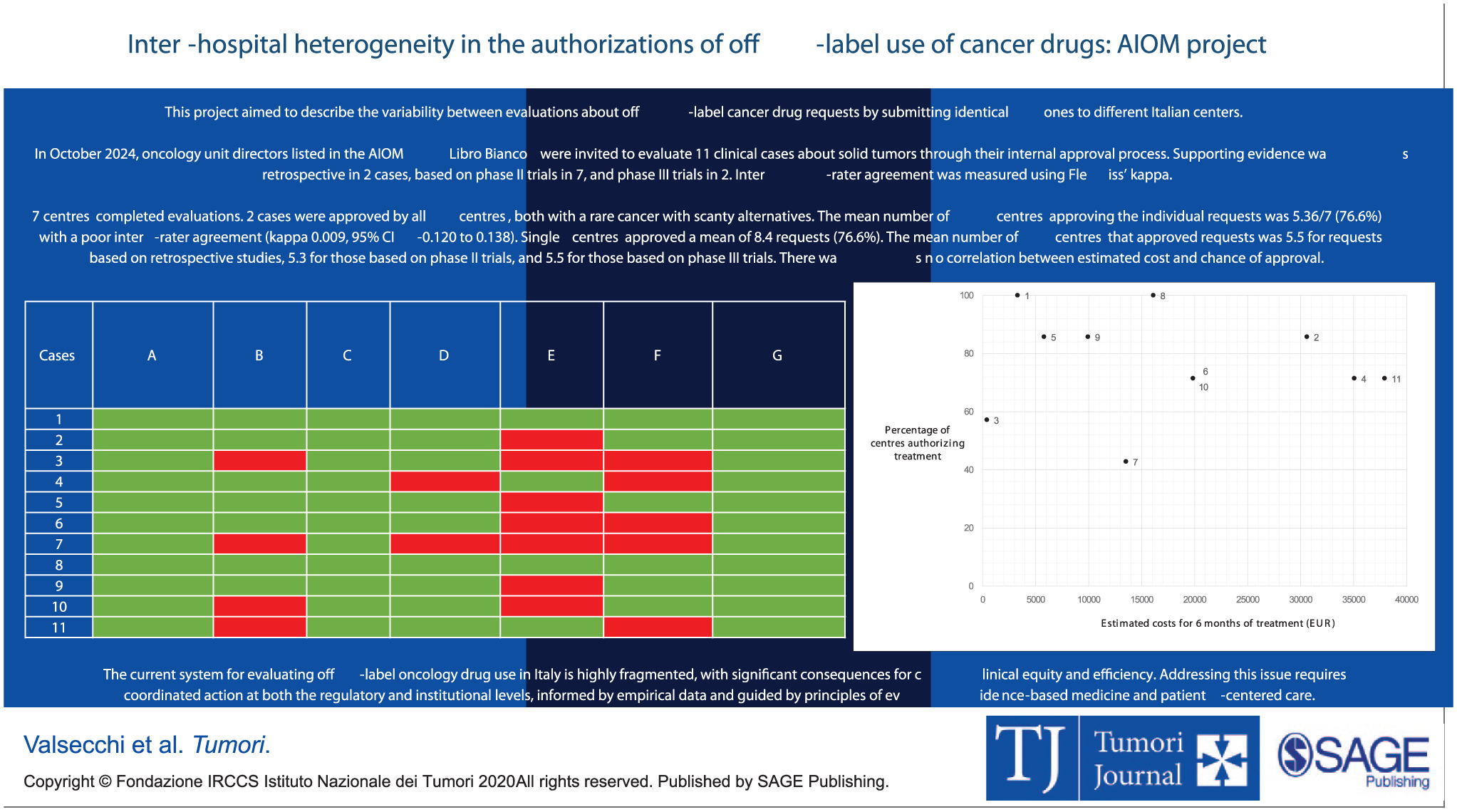
Title: Inter-hospital heterogeneity in the authorizations of off-label cancer drugs: A project by Associazione Italiana di Oncologia Medica
Authors: Anna Amela Valsecchi, Matilde Scaldaferri, Marcello Tiseo, Gianmauro Numico, Gianluca Russo, Nicola Personeni, Livio Blasi, Alessia Pisterna, Alessandra Zanardi, Claudia Fruttero, Giordano Domenico Beretta, Giulia Dusi, Marina Campione, Alessandra Gennari, Maria Rachele Chiappetta, Francesco Perrone, Massimo Di Maio
Read the Full Article on Tumori Journal

“RC48 disitamab vedotin plus toripalimab in mUC positive results in OS and PFS, for patients with expression of HER 2 inclusive 1+, important to build data on her2 + in LATAM.”
“All roads lead to Hamburg Auditorium, Berlin, CityCube A the Tumor Agnostic session kicks off in 15 mins! Grab your espresso and stroll over. You don’t want to miss this.
POWERHOUSE PERSPECTIVES in 90 mins:
- Clinician’s view – Vivek Subbiah
- Statistician’s view – Stefan Michiels
- Pathologist’s view – Fernando Lopez-Rios (Madrid)
- Regulator’s view – Francesco Pignatti (Amsterdam)
Plus 30 min interactive Q&A!”

“Large Ph3 RCT of 294 patients with LA Nasopharyngeal Carcinoma comparing Adj Cap x 6 mo vs observation alone post-CCRT.
As I have said before Cap is great for NPC esp with low disease burden Great work by my previous mentee Dr Miao_JJ from SYSUCC!”

“Dr. Shun Lu delivers HARMONi-6 results at ESMO25: first line ivonescimab + chemotherapy vs tislelizumab (PD-1, approved by China, EMA) + chemo for advanced squamous NSCLC.
Chemotherapy + immunotherapy is our standard of care but squamous outcomes remain poor.”

“FORTITUDE-101
Bemarituzumab (BEMA) + mFOLFOX6 vs PBO + mFOLFOX6 in FGFR2b-overexpressing gastric/GEJ cancer
MOA: BEMA is a first-in-class anti-FGFR2b monoclonal antibody that blocks FGFR2b-driven signaling and triggers ADCC (antibody-dependent cellular cytotoxicity).
Pts: 547 (68.7% ♂, median age 62)
FGFR2b ≥10% 2+/3+ subset → BEMA 159 vs PBO 165
Primary analysis (F/U 11.8 mo):
• mOS 17.9 vs 12.5 mo HR 0.61 (0.43–0.86), p=0.005
• mPFS 8.6 vs 6.7 mo HR 0.71 (0.53–0.95), p=0.019
Follow-up (F/U 19.4 mo):
• mOS 14.5 vs 13.2 mo HR 0.82 (0.62–1.08) to effect attenuated
AEs: G≥3 TEAE 89.5% vs 78.7%; corneal AEs common but manageable
Take: Validates FGFR2b as a new gastric cancer target.
Early OS benefit, later curve convergence → biologically active but not yet practice-changing.
Next: FORTITUDE-102 (BEMA + IO) to test synergy.”

“This AM ESMO25 will be the LBA of STELLAR303 led by our colleagues Drs.Anwaar Saeed and Randy Hecht.”

“At the presidential session with very busy people and investors in GU oncology from Argentina Martín Angel, Fede Losco and the great one, the best time!”

“Congrats to our CUHK Medicine Medical Oncology Fellow Dr. Kingsley Cheung who presented his poster on Real World Longer Term toxicities of selective RET inhibitors and potential targetable mechanisms of resistance at ESMO25 in Berlin!
A large group effort at our institution including investigators, coordinators and research nurses in putting this data collection together.”

“First results from the ADRISK trial (NCT03480672) – Research from the Leipzig University, presented at the oral plenary session: Pembrolizumab added to adjuvant radiochemotherapy in locally advanced HNSCC
Presented at ESMO2025 by Prof. Andreas Dietz (Leipzig, Germany)
Patients with locally advanced head and neck squamous cell carcinoma (HNSCC) often require adjuvant cisplatin-based radiochemotherapy (aRCH) after curative surgery due to intermediate or high-risk pathological features. The phase IIB ADRISK trial investigated whether adding the PD-1 inhibitor pembrolizumab to standard aRCH could improve outcomes.
Study design:
- Randomized 1:1 (n=211, 204 treated)
- Standard aRCH vs. aRCH + pembrolizumab (200 mg q3w for up to 12 months)
- Stratified by p16 status and tumor localization
- Primary endpoint: Event-free survival (EFS)
Key findings (median follow-up: 30 months):
- Numerical improvement in EFS with pembrolizumab (HR 0.81; 95% CI 0.52–1.46; p=0.423)
- No significant difference in OS (HR 0.85; 95% CI 0.46–1.57; p=0.591)
- Subgroup trends:
- HPV-unrelated, CPS ≥10 tumors (≈39% of cohort) showed most pronounced benefit (HR 0.80; 95% CI 0.43–1.48)
- No new safety signals observed
Conclusion:
While ADRISK did not meet its primary endpoint due to fewer than expected events (notably in p16+ cases), the data suggest potential benefit for patients with HPV-unrelated, PD-L1–positive HNSCC — warranting further investigation of adjuvant immunotherapy in this setting.
Congratulations to Prof. Andreas Dietz and the entire ADRISK study group for advancing our understanding of adjuvant immunotherapy in head and neck cancer!”
“Our ESMO Resilience Task Force remains resolute in supporting the wellbeing of oncology professionals worldwide.
We work to evaluate and address burnout, resilience, and wellbeing – our priority is to continue developing practical/sustainable solutions that combine individual and organisational approaches.
At ESMO25, we hosted our annual strategic Task Force meeting, and a Special Forum bringing together experts and frontline professionals to share experiences and feedback to bring this agenda forward.
Watch the space for more to come!”

“Practice-Informing Data in 2L mccRCC | ESMO 2025 Oral Presentation + Annals of Oncology
For the first time, two contemporary second-line options after PD-1–based immunotherapy in metastatic clear cell RCC (mccRCC) have been compared head-to-head:
Lenvatinib + everolimus vs. cabozantinib
LenCabo Phase II Trial Oral presentation at ESMO25
Simultaneous publication in Annals of Oncology
Key Findings
• Median PFS: 15.7 vs 10.2 months (HR 0.51, p = 0.02)
• ORR: 52.6% vs 38.6%
• 1-year OS: 87.0% vs 84.6% (immature)
• Toxicity-related discontinuations: 20% vs 10.9%
Takeaway:
Lenvatinib + everolimus significantly prolonged PFS compared to cabozantinib following progression on PD-1–based ICI combinations.
Proud to be part of the first head-to-head evidence to inform treatment sequencing in the post–IO setting for mccRCC.”
Title: A multicenter randomized phase II trial of lenvatinib plus everolimus versus cabozantinib in patients with metastatic clear cell RCC that progressed on PD-1 immune checkpoint inhibition (LenCabo)
Authors: A.W. Hahn, J. Chahoud, W.P. Skelton, Y. Yuan, A.J. Zurita, C. Kovitz, O. Alhalabi, M.T. Campbell, E. Jonasch, J. Lin, M. Desai, M.J.M.N. Santos, H. Hwang, P.G. Corn, P. Msaouel, N.M. Tannir
Read the Full Article on Annals of Oncology
“ESMO 2025 Day 4: Updates and New Combinations Across HR+, HER2+, and TNBC
Monday, October 20th delivered pivotal insights that will influence treatment sequencing decisions and introduce promising new agents to our armamentarium:
Key HR+/HER2- Advances:
• SONIA Trial OS Analysis: Important academic effort examining CDK4/6i sequencing showed no OS difference between first-line vs second-line use (47.9 vs 48.1 months). While these data provide valuable real-world insights, interpretation requires consideration of patient selection biases and cost-effectiveness implications
• CULMINATE-2: Novel CDK2/4/6 inhibitor culmerciclib + fulvestrant showed impressive PFS benefit (HR 0.56) with manageable toxicity in first-line setting
• PIKALO-1: LY4064809, a pan-mutant-selective PI3Kα inhibitor, demonstrated encouraging activity with notably lower class toxicities – only 1% Grade ≥3 hyperglycemia
Combination Strategies:
• SHR-A1811 + Pertuzumab: Impressive 77.3% ORR in HER2+ mBC with 91% 12-month PFS rate
• BEGONIA Update: Datopotamab deruxtecan + durvalumab achieved remarkable 79-81.8% cORR in first-line TNBC with manageable safety profile.”
ESMO:
“Day 4 -done.
Another day of groundbreaking science and meaningful exchanges at the Congress.
Dive into today’s news and expert insights in the ESMODailyReporter.”

Follow the latest ESMO 2025 news on OncoDaily.
You can also find Part 1 of ESMO 2025.

Part 2 of ESMO 2025.

Part 3 of ESMO 2025.



