HER2 mutations are found in about 2–4% of non–small-cell lung cancers (NSCLC) and are associated with limited targeted treatment options and poor outcomes. While antibody–drug conjugates such as trastuzumab deruxtecan have shown efficacy, safety concerns like interstitial lung disease remain significant. Sevabertinib (BAY 2927088) is an oral, reversible HER2-selective tyrosine kinase inhibitor designed to overcome these limitations.
The phase 1–2 SOHO-01 trial, published in The New England Journal of Medicine in October 2025, evaluated sevabertinib in advanced HER2-mutant NSCLC, demonstrating high response rates, durable activity, and a manageable safety profile across both pretreated and treatment-naïve populations.
Title: Sevabertinib in Advanced HER2-Mutant Non–Small-Cell Lung Cancer
Authors: Xiuning Le, M.D., Ph.D., Tae Min Kim, M.D., Ph.D., Herbert H. Loong, M.B., B.S. , Arsela Prelaj, M.D., Ph.D., Boon Cher Goh, M.B., B.S., Lin Li, M.D., Ph.D., Yong Fang, M.D., Shun Lu, M.D., Ph.D., Xiaorong Dong, M.D., Ph.D., Lin Wu, M.D., Yuki Shinno, M.D., Ph.D., Gennaro Daniele, M.D., Ph.D., Tsung-Ying Yang, M.D., Ph.D., Hye Ryun Kim, M.D., Ph.D., Gerrina Ruiter, M.D., Ph.D., Jun Zhao, M.D., Silvia Novello, M.D., Ph.D., Liyun Miao, Ph.D., Pasi A. Jänne, M.D., Ph.D., Koichi Goto, M.D., Ph.D., Dominik Rüttinger, M.D., Ph.D., Tine Descamps, Ph.D., Jan Christoph Brase, Ph.D., Weichao Bao, Ph.D., Rui Li, M.S., Nicoletta Brega, M.D., Paolo Grassi, M.D., Nicolas Girard, M.D., Ph.D., and Daniel Shao-Weng Tan, Ph.D., M.B., B.S.
Background
HER2 mutations occur in roughly 2–4% of non–small-cell lung cancers (NSCLC) and have historically lacked potent, well-tolerated targeted options. Antibody–drug conjugates (ADCs) such as trastuzumab deruxtecan have activity but carry specific risks (notably interstitial lung disease). Earlier pan-ERBB tyrosine kinase inhibitors (TKIs) produced modest responses (0–19%) and substantial toxicity, prompting development of HER2-selective TKIs that spare wild-type EGFR. Sevabertinib (BAY 2927088) is an oral, reversible TKI with preclinical activity against EGFR/HER2 alterations, including exon 20 insertions (ex20ins). The SOHO-01 trial evaluated sevabertinib in advanced HER2-mutant NSCLC.
Methods
Population. Adults with histologically/cytologically confirmed, locally advanced or metastatic NSCLC harboring activating HER2 mutations were eligible. Key requirements included at least one RECIST v1.1–measurable lesion and ECOG 0–1. Patients with previously treated, asymptomatic brain metastases were eligible; a dedicated cohort enrolled those with active, clinically stable brain metastases (not reported here).
Interventions and Assessments. Sevabertinib was explored across dose levels in dose escalation/backfill, then administered at the recommended phase 2 dose (RP2D) of 20 mg twice daily in expansion/extension phases until progression, unacceptable toxicity, or withdrawal. Responses were assessed by blinded independent central review (BICR) using RECIST v1.1. Time-to-event outcomes used Kaplan–Meier methodology. Safety was graded by CTCAE v5.0.
Primary/Secondary End Points.
- Primary (extension phase): Objective response rate (ORR; complete or partial response) by BICR.
- Secondary: Duration of response (DoR), progression-free survival (PFS), and safety. Exploratory analyses included mutation subtype, coalterations, and circulating tumor DNA (ctDNA) dynamics.
Study Design
SOHO-01 is a multicenter, open-label, phase 1–2 study with dose-escalation and dose-expansion/extension components. At the RP2D (20 mg BID), four HER2-mutant cohorts were defined by treatment history:
- Cohort D: Previously treated; no prior HER2-targeted therapy.
- Cohort E: Previously treated with HER2-directed ADCs
- Cohort F: patients who had not previously received systemic anticancer therapy for locally advanced or metastatic disease
- Cohort G: patients with active, clinically stable brain metastases who had not previously received HER2 ex20ins-targeted tyrosine kinase inhibitors.
- Cohort D1: Previously treated, HER2-targeted–naïve patients who received a lower sevabertinib dose of 10 mg twice daily for dose-comparison purposes.
Enrollment occurred from October 25, 2021, to December 24, 2024. Data cutoff: June 27, 2025.
Results
Patients and Treatment Exposure. A total of 209 patients received sevabertinib 20 mg BID: 81 (Cohort D), 55 (Cohort E), 73 (Cohort F). An additional 36 patients received 10 mg BID (Cohort D1).
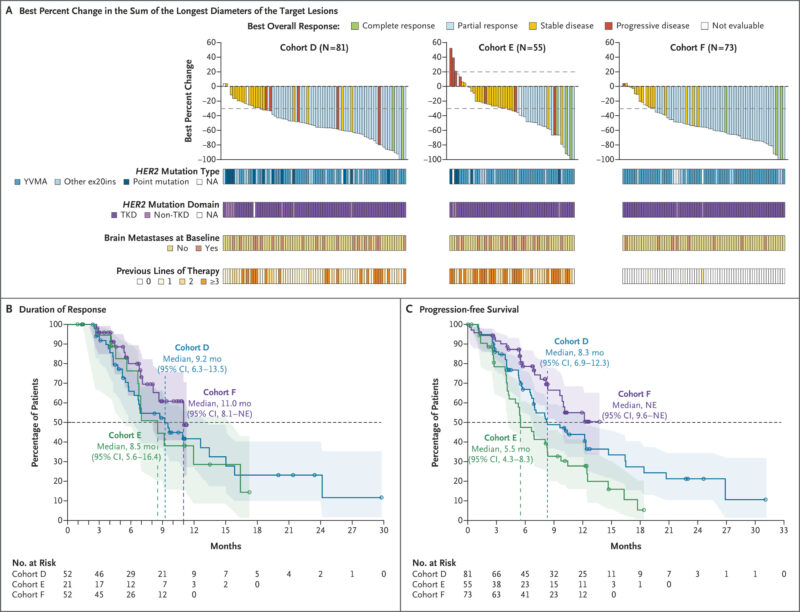
Median follow-up at 20 mg BID was 13.8 months (D), 11.6 months (E), and 9.9 months (F). Treatment was ongoing at cutoff in 30% (D), 25% (E), and 66% (F). Most had HER2 tyrosine kinase domain (TKD) mutations (90–97% across cohorts), with Y772_A775dupYVMA the most prevalent ex20ins (60–79%).
Efficacy (BICR).
- Cohort D (previously treated, HER2-targeted–naïve): ORR 64% (95% CI, 53–75); median DoR 9.2 months (95% CI, 6.3–13.5); median PFS 8.3 months (95% CI, 6.9–12.3).
- Cohort E (post-ADC): ORR 38% (95% CI, 25–52); median DoR 8.5 months (95% CI, 5.6–16.4); median PFS 5.5 months (95% CI, 4.3–8.3). Among 41 prior trastuzumab deruxtecan recipients, ORR 34% (95% CI, 20–51).
- Cohort F (treatment-naïve): ORR 71% (95% CI, 59–81); median DoR 11.0 months (95% CI, 8.1–NE). PFS immature at cutoff.
- Cohort D1 (10 mg BID): ORR 36% (95% CI, 21–54); DoR 6.9 months (95% CI, 4.1–15.0); PFS 5.6 months (95% CI, 4.1–12.3) by investigator review.
- Brain Metastases. Among patients with baseline brain metastases, responses were observed in 61% (D), 27% (E), and 78% (F). Among those without baseline CNS disease, only 5% (8/167) had CNS as the first site of progression.
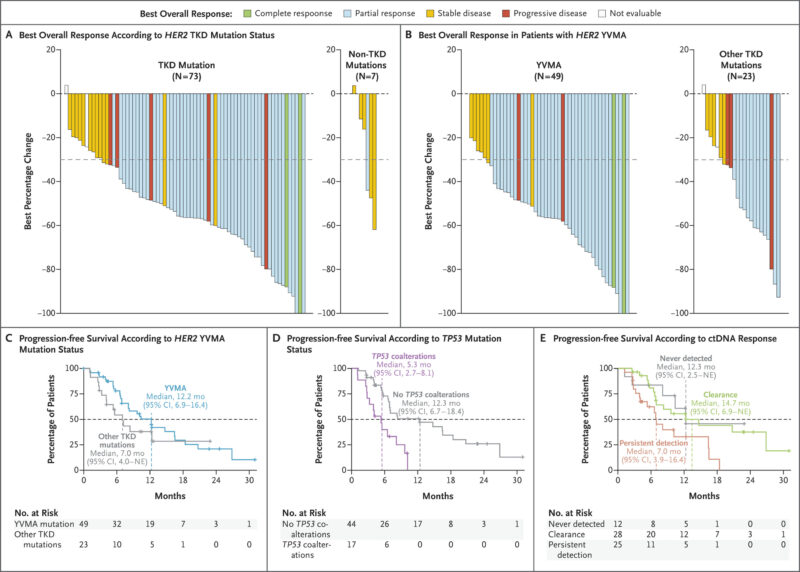
Biomarker/Genomic Signals (Cohort D).
- TKD vs non-TKD: TKD mutations showed higher activity (ORR 70% vs 14%) and longer PFS (9.6 vs 6.9 months).
- Y772_A775dupYVMA: Higher ORR 78% and longer PFS 12.2 months versus other HER2 TKD alterations (ORR 57%; PFS 7.0 months).
- ctDNA dynamics (baseline to 2–6 weeks): “Never detected” and “clearance” subgroups had prolonged PFS (12.3 and 14.7 months) compared with “persistent detection” (7.0 months).
- Coalterations: TP53 mutations (28% of detectable HER2-ctDNA cases) associated with shorter PFS (5.3 vs 12.3 months without TP53 coalterations).
Safety.
Drug-related adverse events (AEs) were common but manageable, with grade ≥3 drug-related AEs in 23–38% across cohorts at 20 mg BID.
- Diarrhea was most frequent: 84–91% any-grade; grade 3 in 23% (D), 11% (E), 5% (F). No grade 4 diarrhea; no discontinuations due to diarrhea; dose reductions for diarrhea in 7–12%.
- Rash occurred in 47–51%; paronychia in 22–29%.
- Serious drug-related AEs occurred in 8–15%; discontinuations due to drug-related AEs were 1–5%.
- Interstitial lung disease/pneumonitis was not reported as drug-related.
- Single grade 5 drug-related event: cardiorespiratory arrest (one patient).
Key Findings
This large phase 1–2 experience (n=209 at the RP2D) shows that sevabertinib produces clinically meaningful response rates across clinically relevant settings of HER2-mutant NSCLC:
- Previously treated, HER2-targeted–naïve (D): ORR 64%, median PFS 8.3 months, DoR 9.2 months.
- Post-ADC (E): ORR 38%, PFS 5.5 months, DoR 8.5 months; maintained activity after trastuzumab deruxtecan (ORR 34%).
- First-line (F): ORR 71% and DoR 11.0 months with immature PFS.
- Genomics: The Y772_A775dupYVMA ex20ins subgroup derived particularly strong benefit (ORR 78%, PFS 12.2 months). TP53 coalterations portended shorter PFS. Early ctDNA clearance/undetectable status associated with prolonged PFS.
- Safety: Diarrhea, rash, and paronychia were the leading AEs; grade ≥3 diarrhea was cohort-dependent but manageable, and no drug-related ILD was observed.
Conclusion
In the phase 1–2 SOHO-01 study, sevabertinib (20 mg twice daily) achieved ORR 64% in previously treated, HER2-targeted–naïve patients, 38% after prior HER2-directed ADCs, and 71% in treatment-naïve patients, with DoR of 8.5–11.0 months across cohorts and PFS 5.5–8.3 months where mature. Activity was particularly notable in HER2 TKD alterations and the Y772_A775dupYVMA subtype.
Diarrhea was the most frequent adverse event; grade ≥3 toxicity occurred in roughly one-quarter to one-third of patients, and no drug-related ILD was reported. Together, these findings support sevabertinib as a clinically relevant option for HER2-mutant NSCLC across multiple treatment settings and justify phase 3 confirmation (SOHO-02).
You can read the full article here.


