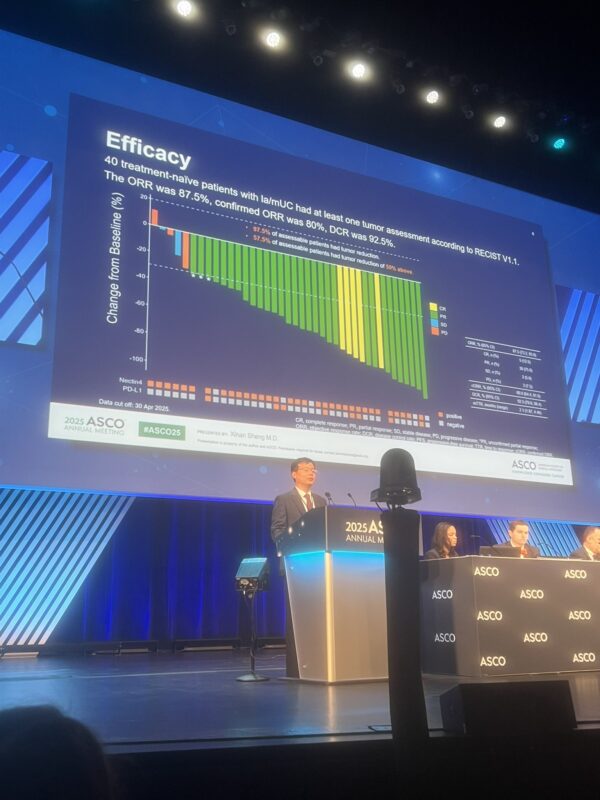
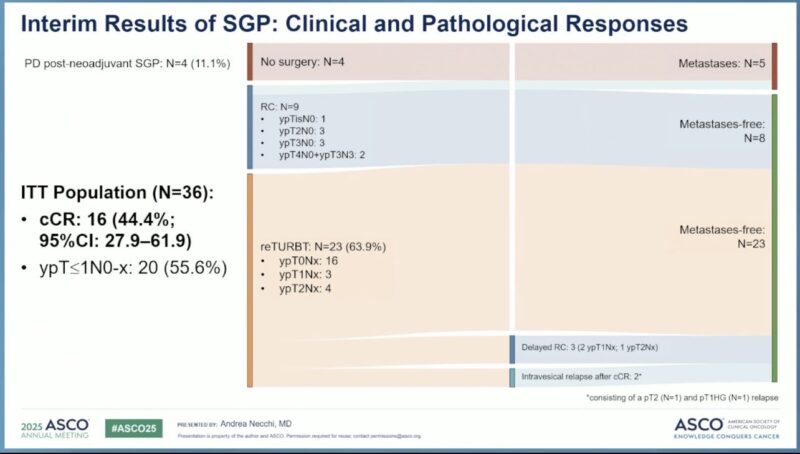
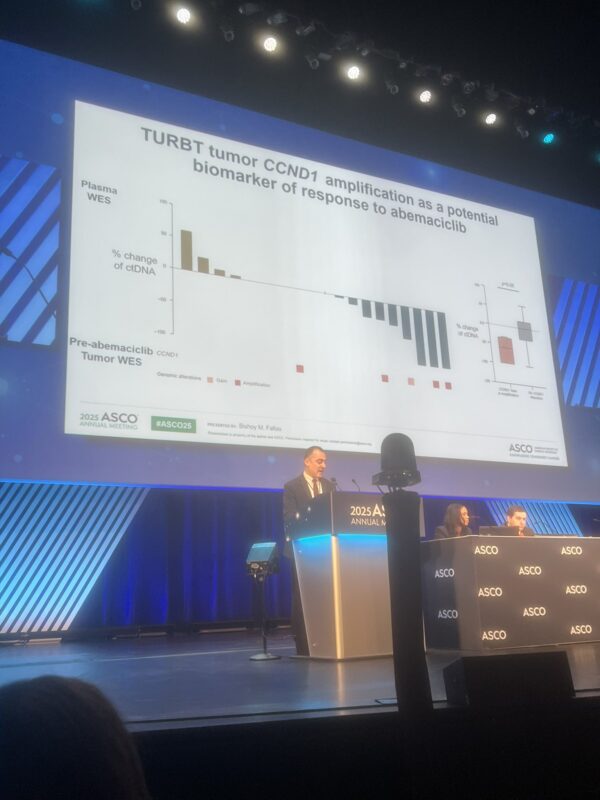
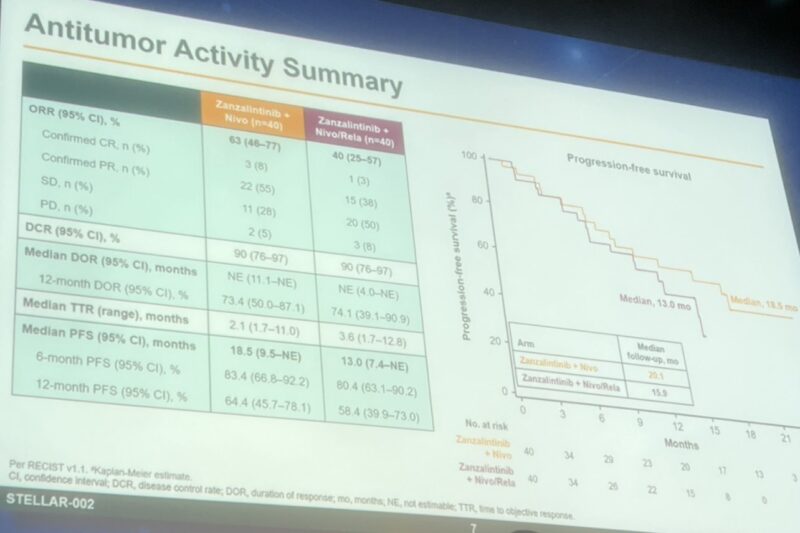
The ASCO Annual Meeting 2025 is now in full swing in Chicago! From May 30 to June 3, cancer experts, researchers, and advocates from around the globe are gathering to exchange ideas, unveil new discoveries, and drive forward the future of cancer care.
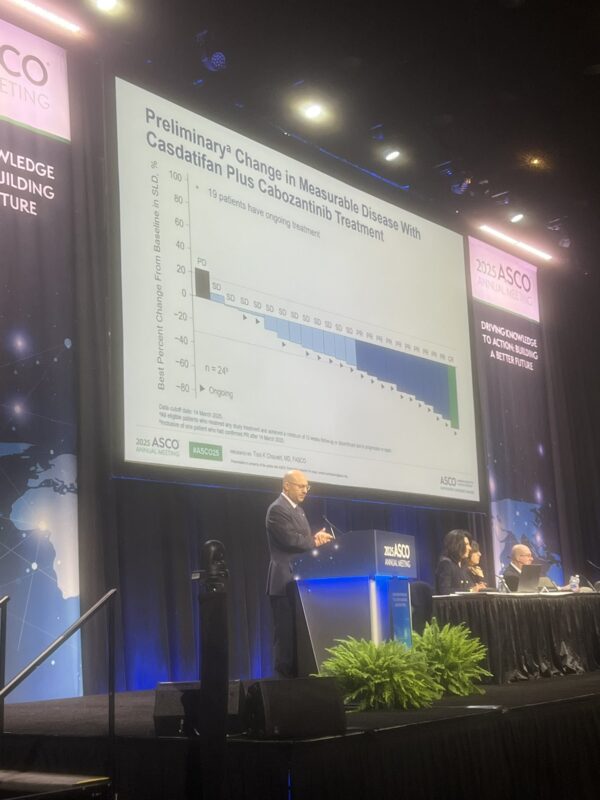
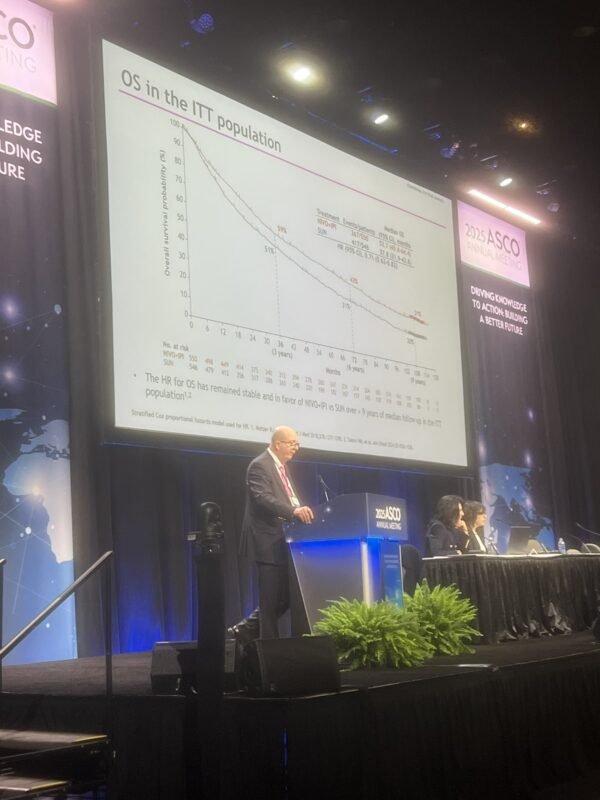
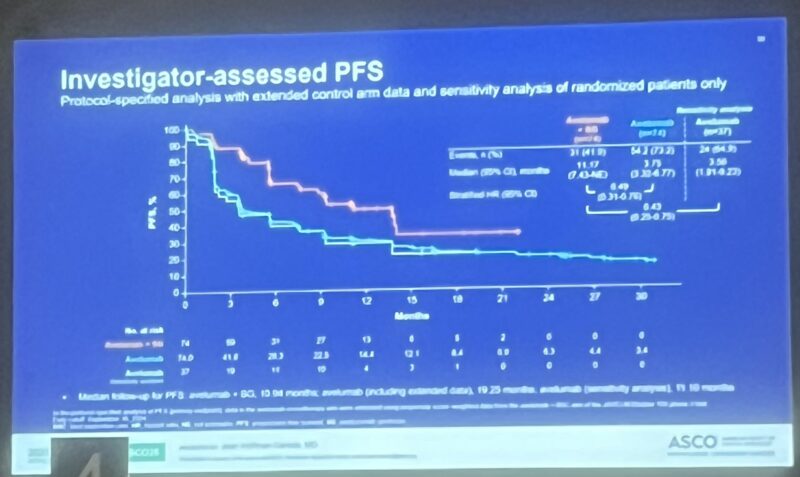
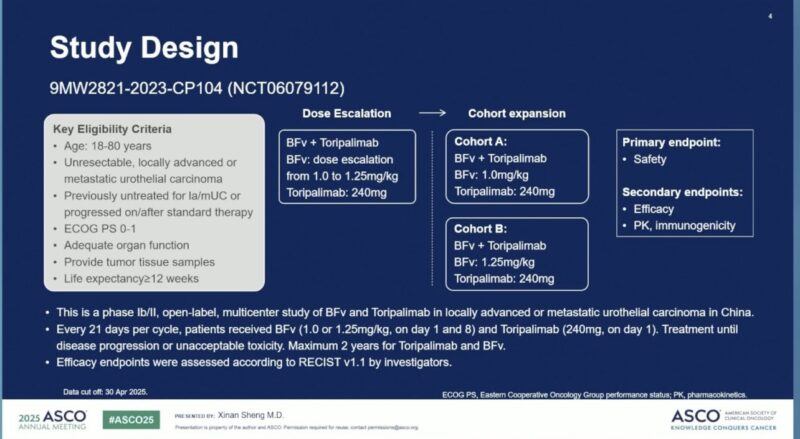
Dr. Tom Powles, a leading voice in genitourinary oncology, offered a steady stream of updates from ASCO25 that captured both the excitement and the complexity of evolving therapies in RCC, UC, and MIBC.
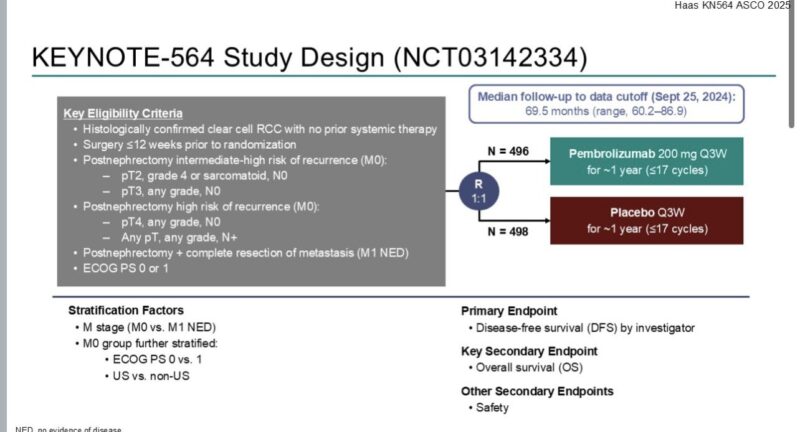
Here’s a roundup of his most notable takeaways:
These snapshots from Dr. Tom Powles reflect a field in flux—defined by innovation, nuanced decisions, and a constant recalibration of what progress truly means. As new agents push into earlier lines of treatment, the oncology community is watching closely.
Stay tuned to OncoDaily for more updates from ASCO25.