Amivantamab-Lazertinib Improves Outcomes in High-Risk EGFR-Mutant NSCLC Subgroups: MARIPOSA Study Results
Authors: E. Felip, B.C. Cho, V. Gutiérrez, A. Alip, B. Besse, S. Lu, A.I. Spira, N. Girard, R. Califano, S.M. Gadgeel, J.C.-H. Yang, S. Yamamoto, K. Azuma, Y.J. Kim, K.-H. Lee, P. Danchaivijitr, C.G. Ferreira, Y. Cheng, M.A.N. Sendur, G.-C. Chang, C.-C. Wang, K. Prabhash, Y. Shinno, D. Stroyakovskiy, L. Paz-Ares, J.R. Rodriguez, C. Martin, M. R. G. Campelo, H. Hayashi, D. Nguyen, P. Tomasini, M. Gottfried, C. Dooms, A. Passaro, M. Schuler, A.C. Z. Gelatti, S. Owen, K. Perdrizet, S.-H. I. Ou, J.C. Curtin, J. Zhang, M. Gormley, T. Sun, A. Panchal, M. Ennis, E. Fennema, M. Daksh, S. Sethi, J.M. Bauml, S.-H. Lee.
Published in Annals of Oncology on June 26, 2024 ,
Introduction:
Advanced non-small cell lung cancer (NSCLC) patients often present with high-risk features like detectable circulating tumor DNA (ctDNA), TP53 co-mutations, and liver or brain metastases. These characteristics are associated with poorer outcomes, especially when treated with EGFR tyrosine kinase inhibitors (TKIs) like osimertinib. The MARIPOSA study investigated the efficacy of amivantamab (an EGFR-MET bispecific antibody) combined with lazertinib (a third-generation EGFR TKI) compared to osimertinib in treatment-naïve EGFR-mutant advanced NSCLC patients, including those with high-risk features.
Design/Methods:
The phase III MARIPOSA study enrolled 1074 treatment-naïve adults with EGFR Ex19del or L858R mutated advanced NSCLC. Patients were randomized 2:2:1 to receive amivantamab-lazertinib (n=429), osimertinib (n=429), or lazertinib monotherapy (n=216). This analysis focused on high-risk subgroups receiving amivantamab-lazertinib or osimertinib.
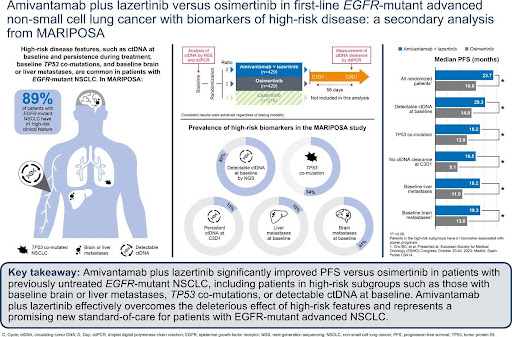
Key assessments included:
- ctDNA analysis by next-generation sequencing (NGS) using Guardant360 CDx at baseline
- EGFR-mutant ctDNA detection and clearance by Biodesix droplet digital PCR (ddPCR) at baseline and Cycle 3 Day 1 (C3D1)
- Presence of liver metastases at baseline
- Progression-free survival (PFS) in various subgroups
What We Learned:
- ctDNA and TP53 co-mutations:
- 85% of patients had detectable ctDNA at baseline by NGS
- 54% of patients with analyzable baseline ctDNA had TP53 co-mutations
- Amivantamab-lazertinib significantly improved PFS vs osimertinib in patients with: a) Detectable baseline ctDNA (20.3 vs 14.8 months; HR 0.71, P=0.003) b) TP53 co-mutations (18.2 vs 12.9 months; HR 0.65, P=0.003)
- EGFR-mutant ctDNA by ddPCR:
- Amivantamab-lazertinib significantly prolonged PFS vs osimertinib in patients with: a) Detectable baseline EGFR-mutant ctDNA (20.3 vs 14.8 months; HR 0.68, P=0.002) b) No ctDNA clearance at C3D1 (16.5 vs 9.1 months; HR 0.49, P=0.015) c) ctDNA clearance at C3D1 (24.0 vs 16.5 months; HR 0.64, P=0.004)
- Liver metastases:
- 16% of patients had liver metastases at baseline
- Amivantamab-lazertinib significantly improved PFS vs osimertinib in patients: a) With liver metastases (18.2 vs 11.0 months; HR 0.58, P=0.017) b) Without liver metastases (24.0 vs 18.3 months; HR 0.74, P=0.004)
- High-risk subgroup analysis:
- 89% of patients with analyzable baseline ctDNA had at least one high-risk feature
- Amivantamab-lazertinib significantly prolonged PFS vs osimertinib in patients with any high-risk feature (20.3 vs 15.0 months; HR 0.72, P=0.004)
Key Highlights:
- Amivantamab-lazertinib consistently demonstrated superior PFS compared to osimertinib across all high-risk subgroups analyzed.
- The combination therapy was particularly effective in patients with liver metastases, potentially due to amivantamab’s MET activity.
- Patients without ctDNA clearance at C3D1 had the shortest PFS among all subgroups, but amivantamab-lazertinib still significantly improved outcomes compared to osimertinib.
- The median PFS for osimertinib in high-risk subgroups was notably shorter than in the overall MARIPOSA population, emphasizing the need for more effective first-line treatments in these patients.
Key Takeaway Messages:
- Amivantamab-lazertinib effectively overcomes the negative prognostic impact of high-risk features in EGFR-mutant advanced NSCLC.
- The combination therapy represents a promising new standard-of-care option for patients with EGFR-mutant advanced NSCLC, particularly those with high-risk characteristics.
- The study underscores the importance of using the most efficacious therapy first, especially in patients with poor prognostic factors.
- ctDNA analysis and monitoring may provide valuable insights for treatment decision-making and prognosis in EGFR-mutant NSCLC.
- Further research is needed to understand the mechanisms behind the improved efficacy of amivantamab-lazertinib in high-risk subgroups and to optimize treatment strategies for these patients.
MARIPOSA study demonstrates that amivantamab-lazertinib significantly improves outcomes in patients with EGFR-mutant advanced NSCLC, including those with high-risk features such as detectable ctDNA, TP53 co-mutations, and liver metastases. These findings support using this combination therapy as a new standard-of-care option for this patient population.
Summary by Amalya Sargsyan, MD


