Targeting NECTIN4. Predicting Response to Enfortumab Vedotin in Metastatic Urothelial Cancer
Authors: Niklas Klümper, MD, Ngoc Khanh Tran, Stefanie Zschäbitz, MD, Oliver Hahn, MD, Thomas Büttner, MD, Florian Roghmann, MD, Christian Bolenz, MD, Friedemann Zengerling, MD, Constantin Schwab, MD, Dora Nagy, MD, Marieta Toma, MD, Glen Kristiansen, MD, Hendrik Heers, MD, Philipp Ivanyi, MD, Günter Niegisch, MD, Camilla Marisa Grunewald, MD, Christopher Darr, MD, Arian Farid, MD, Katrin Schlack, MD, Mahmoud Abbas, MD, Can Aydogdu, MD, Jozefina Casuscelli, MD, Theresa Mokry, MD, Michael Mayr, MD, Dora Niedersüß-Beke, MD, Steffen Rausch, MD, Dimo Dietrich, PhD, Jonas Saal, MD, Jörg Ellinger, MD, Manuel Ritter, MD, Abdullah Alajati, PhD, Christoph Kuppe, MD, PhD, Joshua Meeks, MD, PhD, Francisco E. Vera Badillo, MD, J. Alberto Nakauma-González, PhD, Joost Boormans, MD, Kerstin Junker, MD, Arndt Hartmann, MD, Viktor Grünwald, MD, Michael Hölzel, MD, PhD, and Markus Eckstein, MD.
Published in the Journal of Clinical Oncology on April 24, 2024
Introduction:
Enfortumab vedotin (EV), an antibody-drug conjugate (ADC) targeting NECTIN4, has been approved for the treatment of metastatic urothelial cancer (mUC). However, durable responses are only observed in a small subset of patients. This study evaluated NECTIN4 amplifications as a potential genomic biomarker to predict EV response in patients with mUC.
Design and Methods:
The researchers established a NECTIN4-specific fluorescence in situ hybridization (FISH) assay to assess the predictive value of NECTIN4 copy number variations (CNVs) in a multicenter cohort of 108 EV-treated mUC patients. CNVs were correlated with membranous NECTIN4 protein expression, EV treatment responses, and outcomes. The study also assessed the prognostic value of NECTIN4 CNVs in a non-EV-treated mUC cohort (n=103) and queried The Cancer Genome Atlas (TCGA) data sets for NECTIN4 CNVs across 32 cancer types.
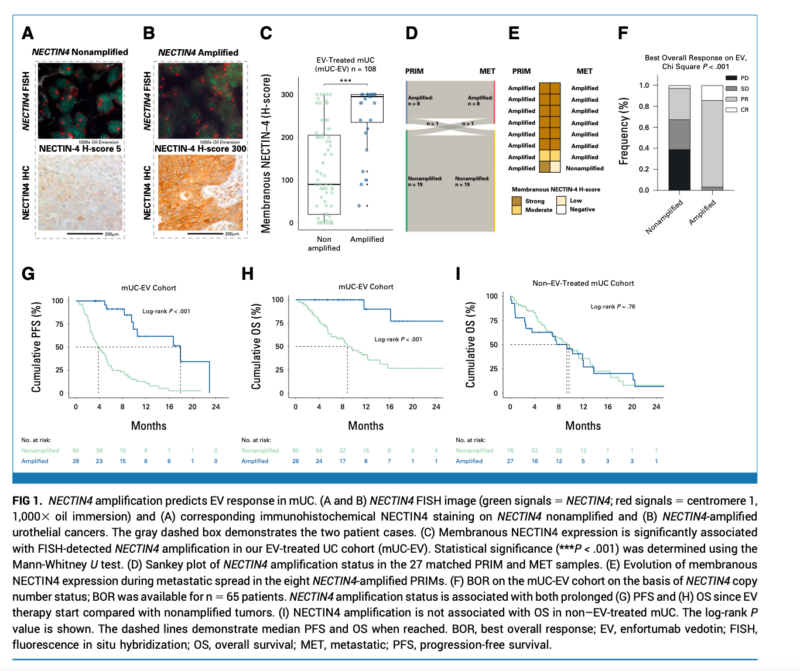
What We Learned:
- NECTIN4 amplifications are frequent genomic events occurring in approximately 26% of mUC cases and 17% of TCGA bladder cancer cases.
- In the EV-treated mUC cohort, 96% of patients with NECTIN4 amplifications demonstrated objective responses to EV, compared to 32% in the non-amplified subgroup (p<0.001).
- In the EV-treated cohort, NECTIN4 amplifications were associated with prolonged progression-free survival (PFS) and overall survival (OS). In multivariable analysis, NECTIN4 amplification led to a 92% risk reduction for death (HR 0.08, 95% CI 0.02-0.34, p<0.001).
- NECTIN4 amplifications were not associated with outcomes in the non-EV-treated mUC cohort, suggesting they are a predictive biomarker rather than a prognostic factor.
- NECTIN4 amplifications were observed across various solid tumor types, with frequencies ranging from 5-10% in breast and lung cancers.
Key Highlights:
- NECTIN4 amplifications are stable genomic alterations associated with enhanced membranous NECTIN4 protein expression and predict exceptional and durable responses to EV in mUC.
- NECTIN4 FISH assay could be easily implemented in clinical trials and routine molecular pathology for biomarker-guided EV therapy.
- The frequent occurrence of NECTIN4 amplifications across cancer types suggests potential tumor-agnostic clinical development of NECTIN4-targeted ADCs based on this biomarker.
Key Takeaway Messages:
- NECTIN4 amplifications predict response to enfortumab vedotin in metastatic urothelial cancer.
- NECTIN4 FISH assays enable biomarker-guided patient selection for enfortumab vedotin.
- NECTIN4 amplifications occur across solid tumors like breast and lung cancers – a potential tumor-agnostic biomarker.
- Prospective validation of NECTIN4 amplification as a predictive biomarker is needed.
- NECTIN4 amplification stability during metastasis suggests a durable response mechanism.
- Biomarker-driven trials could enable tumor-agnostic enfortumab vedotin development.
Summary by Amalya Sargsyan, MD


