Madelon de Jong, a Researcher at the Kennedy Institute of Rheumatology, shared a post on X:
“My final PhD paper is out in Nature Immunology.
We previously identified inflammatory stroma in the myeloma marrow. But what is it doing there? Here, we show it conspires with the most abundant marrow cell for long-term tumor support: the neutrophil!
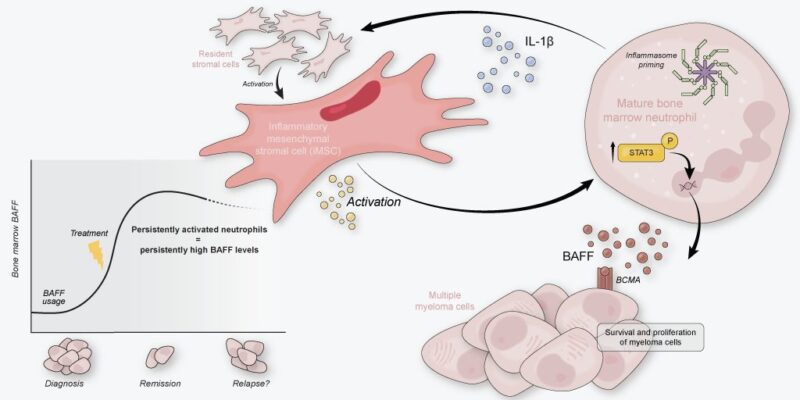
Neutro infiltration is a hallmark of many solid tumor microenvironments, where they can create pro-tumor havoc. The bone marrow (BM) permanently harbors neutrophils, so a pro-tumor bias of these cells would have huge potential to influence the pathobiology of BM malignancies.
We had previously found that the newly diagnosed MM BM is characterized by inflammatory mesenchymal stromal cells (iMSC). These iMSC transcribed a myriad of neutrophil modulation factors, in addition to tumor-survival factors such as IL-6.
Therefore, we hypothesized that stromal-neutrophil interactions could significantly impact the tumor-supportive tumor microenvironment in MM.
Are neutros still there in diseased BM? Yes, they are! Unlike leukemia, MMSM patients rarely present with neutropenia. We performed scRNA seq of the neutrophil lineage of 6 NDMM patients and 4 controls and observed a cluster of mature neutrophils (no. 2) to be enriched in NDMM BM.
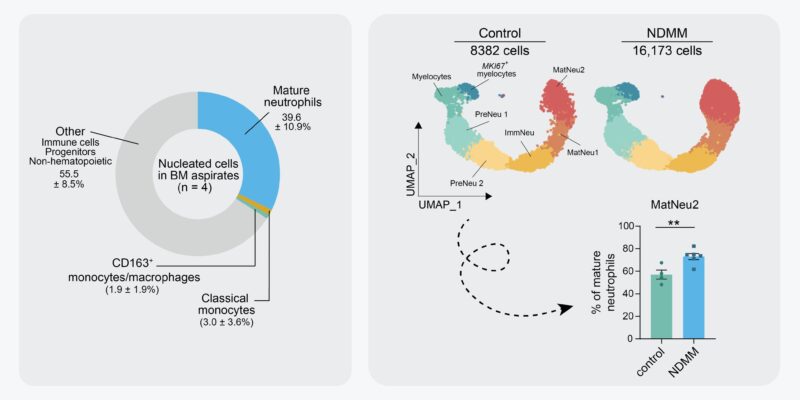
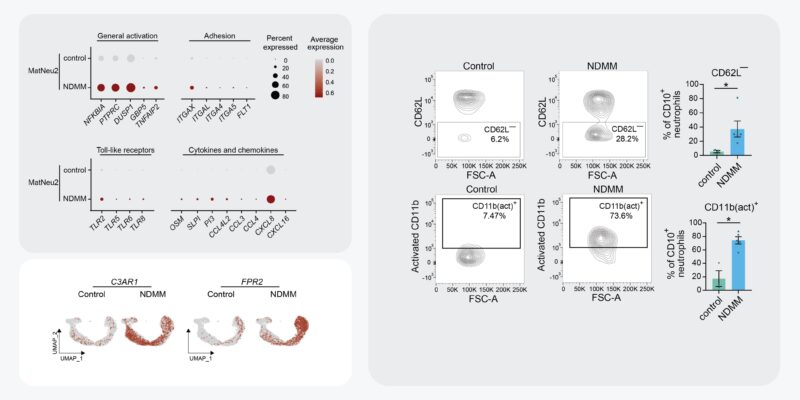
These enriched mature neutros were characterized by increased transcription of genes associated with neutrophil activation and inflammation, including, inflammatory cytokines, pattern-recognition receptors, and integrins. We validated neutro activation in MM BM by flow cytometry.
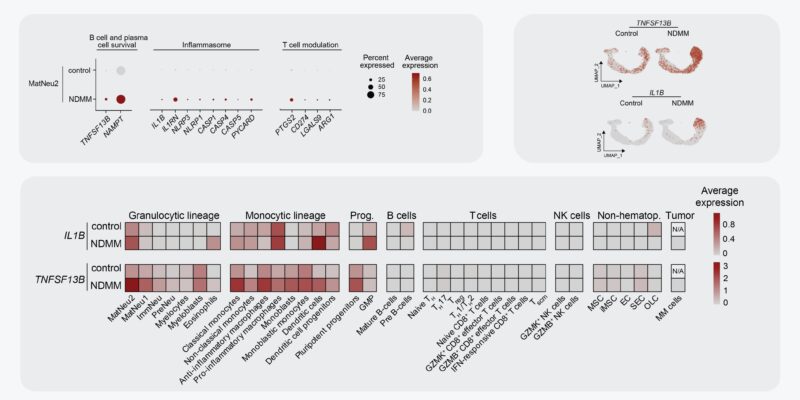
Activated neutros also transcribed increased levels of genes associated with MM pathobiology, including TNFSF13B (= BCMA-ligand BAFF) and IL1B. Using our in-house MM scRNA seq datasets, we show that activated neutros are a dominant source of these transcripts in MM BM!
As iMSC transcribe neutro modulation factors, can they induce activation of neutros in the MM BM? To study iMSC – neutro interactions, we developed an in vitro co-culture model using IL-1β pre-stimulated primary BMSC to obtain iMSC-like cells and blood neutros of healthy donors.
When naive neutros were co-cultured with iMSC-like cells, they obtained activated transcriptomes, increased activation surface markers, and started secreting BAFF and IL-1β. All in all, iMSC-like cells induced neutros to become VERY similar to those we see in MM BM.
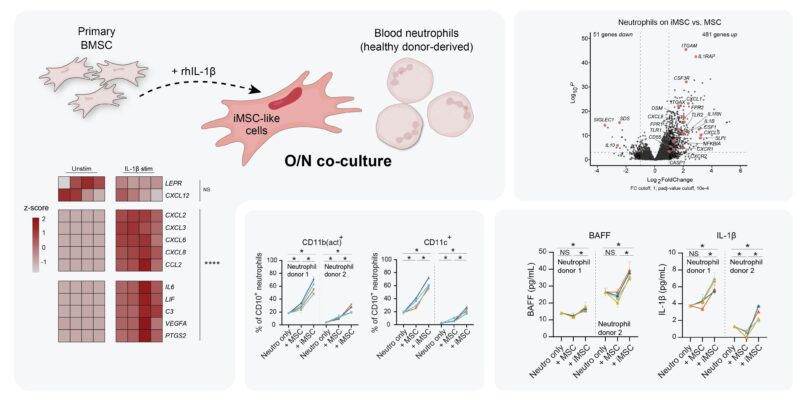
In MM patients, we saw signs of STAT3-signaling activated neutros. In vitro, iMSC-induced neutros also showed feats of STAT3 signaling. Adding STAT3 inhibitor Stattic to co-cultures determined that iMSC-induced activation of neutros incl BAFF secretion was indeed STAT3-dependent.
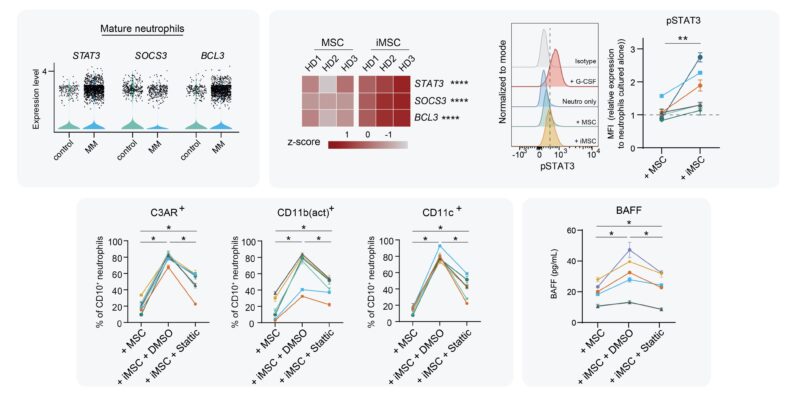
As iMSC produces IL-6, which signals via STAT3, we hypothesized IL-6 to be the culprit. To our surprise, neutralization of IL-6 in neutro-iMSC co-cultures did not block the activation of neutros, nor did IL-6 stim of neutros activate them.

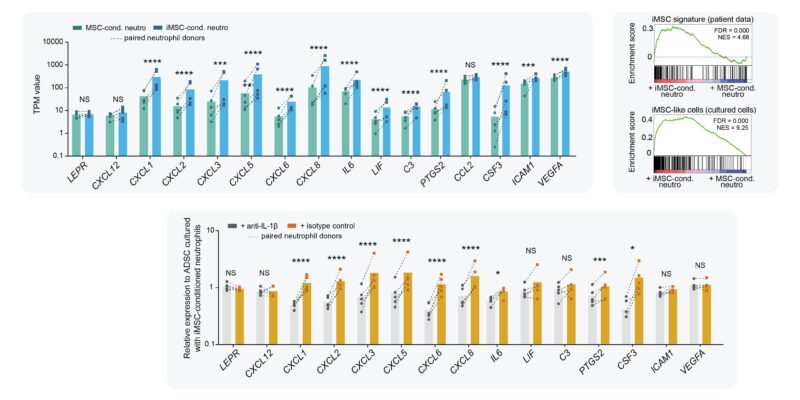
In contrast to MSC, neutros are motile cells that could potentially spread stromal inflammation. Therefore, we tested whether iMSC-activated neutros gain the ability to de novo activate naive stromal cells. Turns out they do!
When we transferred iMSC-conditioned neutros to naive stromal cells for O/N co-culture, naive stromal cells became activated and obtained an iMSC-like transcriptome. By neutralizing IL-1β during co-culture, we showed that this process was (mostly) IL-1β-dependent.
So stromal-neutro interactions steer BM inflammation in newly diagnosed mmsm. What happens after treatment? We investigated the presence of iMSC and activated neutros in MM BM after 1st-line treatment, including induction, HD melphalan, ASCT, and consolidation w/ triplet +/- anti-CD38.
At diagnosis, stromal inflammation is so strong that iMSC forms separate clusters when scRNA-seqing MSC. This is not the case anymore in treated BM! Sadly though, iMSC are not gone, demonstrated by continued transcription of iMSC genes and high percentages of CD44+ iMSC by flow.

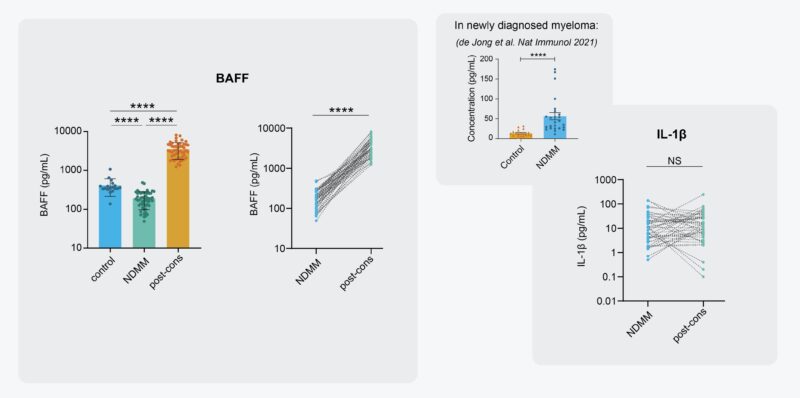
Regarding neutros, they’re still the most abundant cell type in treated BM. Moreover, scRNA seq demonstrated neutros are still activated post 1st-line treatment, and that these neutros are still significant sources of pro-tumor transcripts BAFF and IL1B.

Finally, we measured BAFF and IL-1β in paired BM plasma samples of 52 patients at diagnosis and after treatment. Both proteins are still strongly elevated in treated BM. The consequence for disease recurrence? (The low levels of BAFF diagnosis are due to consumption by the tumor!)
Our work identifies neutros as core components of the MM tumor microenvironment by providing BAFF and IL-1β. We conceptualize that neutro activation is mediated by MM-specific iMSC, creating an activation loop that persists after treatment.
This merits investigation into its role in disease relapse, and also suggests both compartments should be targeted to achieve the best possible therapy response in patients.
This work will form the final chapter of my PhD thesis (FINALLY! Dutch rules suck). I’m exceptionally proud of it, lots of blood/sweat/tears, lots of setbacks, but we got there. A great final project together with my PhD advisor and mentor Tom Cupedo.
My Erasmus MC Hematology colleagues were indispensable throughout this work, Cathelijne Fokkema, Sabrin Tahri, Natalie, Mark, and Zoltán Kellermayer. Thank you and I miss you! Also big thanks to Pieter Sonneveld, Mathijs Sanders, Heiko Bruns, and all other co-authors.
Thanks to the reviewers who helped improve the final print! And finally (but importantly), thanks to the patients for donated samples (which is what this type of work heavily relies on).
This study was funded by the Myeloma Society Paula and Rodger Riney Foundation translational research award (for Tom Cupedo) and grants from the EMN_EuMMnet. Thanks all!
Read further.
Source: Madelon de Jong/X


