Zenocutuzumab (also known as Zeno or MCLA-128) is a new cancer treatment that targets a specific gene fusion, NRG1, found in certain types of cancer. Approved by the U.S. Food and Drug Administration (FDA), Zenocutuzumab offers new hope for patients with advanced cancers, including non-small cell lung cancer (NSCLC) and pancreatic cancer. Here’s everything you need to know about Zenocutuzumab, how it works, and what you can expect if you’re prescribed this treatment.
What Is Zenocutuzumab and How Does It Work?
Zenocutuzumab is a type of targeted therapy called a bispecific monoclonal antibody. This drug is designed to specifically target and block signals that cause cancer cells to grow, especially in cancers with a genetic change called NRG1 fusion. By blocking two receptors, HER2 and HER3, that are involved in cancer cell growth, Zenocutuzumab stops the cancer from growing and spreading.
In addition to targeting cancer cells directly, Zenocutuzumab helps your immune system recognize and destroy cancer cells more effectively. It works by recruiting immune cells to the tumor site, helping the body’s natural defenses fight the cancer.
What Company Produces Zenocutuzumab?
Zenocutuzumab is developed by Merus N.V., a clinical-stage biotechnology company based in Utrecht, the Netherlands. Merus specializes in creating innovative antibody-based therapies designed to treat various forms of cancer. The company has been at the forefront of research in bispecific antibodies, which can engage multiple targets simultaneously, enhancing the effectiveness of cancer treatment.
Is Zenocutuzumab Approved for Use?
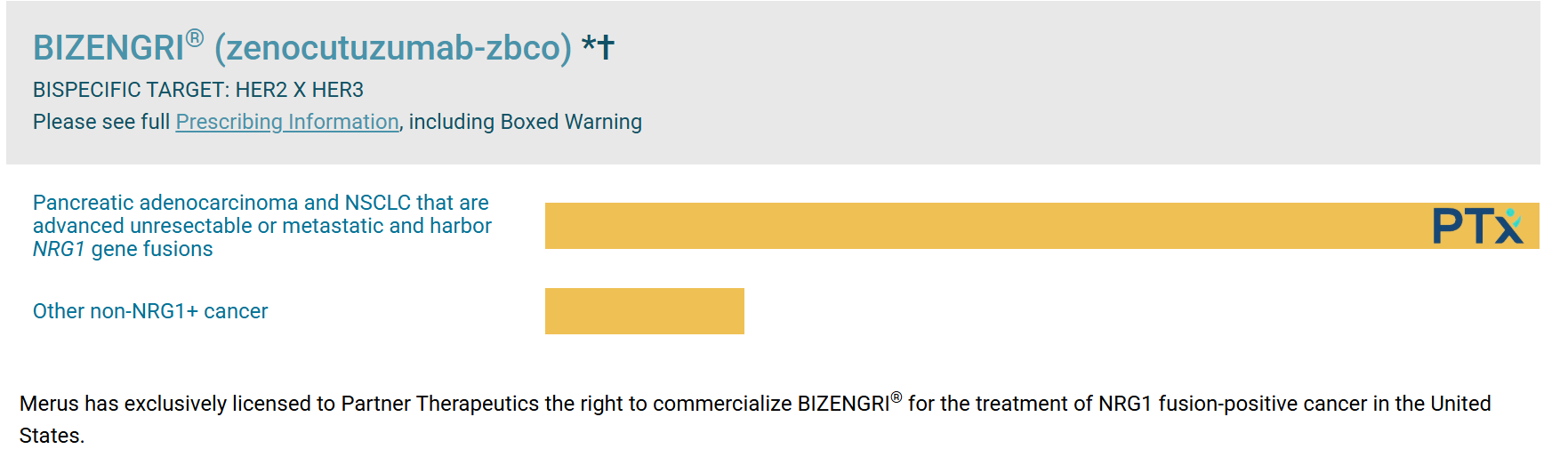
Yes, Zenocutuzumab has been FDA-approved for the treatment of certain types of advanced cancer. On December 4, 2024, the FDA granted accelerated approval for Zenocutuzumab under the brand name Bizengri. It is approved for adults with advanced, unresectable, or metastatic non-small cell lung cancer (NSCLC) and pancreatic adenocarcinoma that harbor the NRG1 gene fusion. This approval was based on promising results from clinical trials showing that Zenocutuzumab is effective in treating these specific cancers, particularly after other treatments have not worked.

What Is a Clinical Trial and What Does It Mean That Zenocutuzumab Was Approved?
A clinical trial is a research study in which new treatments are tested to see if they are safe and effective. Zenocutuzumab was tested in clinical trials where it showed good results for people with cancers containing the NRG1 gene fusion.
When we say Zenocutuzumab was FDA-approved, it means that the treatment has met the necessary standards for safety and effectiveness and is now available for doctors to prescribe. This approval gives patients a new treatment option for cancers that are hard to treat.
What Cancers Can Zenocutuzumab Treat?
Zenocutuzumab is approved to treat advanced or metastatic non-small cell lung cancer (NSCLC) and pancreatic cancer in patients who have the NRG1 gene fusion. This gene fusion is found in a small but specific group of patients with these cancers. Zenocutuzumab targets and blocks the growth of tumors caused by this gene fusion.
What Do Recent Studies Show About Zenocutuzumab’s Effectiveness?
Recent studies have shown that Zenocutuzumab has demonstrated promising results in treating cancers with NRG1 gene fusion, particularly in non-small cell lung cancer (NSCLC) and pancreatic cancer. Here’s what we know about its effectiveness:
-
In Lung Cancer: In the eNRGy Phase 2 Trial (NCT02912949), Zenocutuzumab was tested in patients with advanced non-small cell lung cancer (NSCLC) that had progressed after previous treatments. The drug showed a 29% response rate in lung cancer patients, meaning almost a third of patients experienced a reduction in their tumor size. The median duration of response was 11.1 months, meaning these patients maintained their tumor shrinkage for over 10 months on average.
-
In Pancreatic Cancer: Pancreatic cancer is known for being particularly hard to treat, but Zenocutuzumab showed a 42% response rate in patients with NRG1 fusion-positive pancreatic adenocarcinoma. These patients experienced tumor shrinkage and improved progression-free survival (PFS), which was 6.8 months on average. This is a significant improvement compared to traditional treatments, which often show much shorter times before cancer progression.
How Does Zenocutuzumab Compare to Other Treatments?
In clinical trials, Zenocutuzumab has shown efficacy comparable to or even better than other existing treatments for NSCLC and pancreatic cancer with NRG1 gene fusions. While most traditional therapies may not target these specific gene fusions, Zenocutuzumab was developed with this precise issue in mind. Its ability to block HER2 and HER3 receptors and stop the signaling that drives cancer cell growth makes it a unique option for patients who haven’t responded well to chemotherapy or other targeted therapies.

Zenocutuzumab was recognized among 10 most promising cancer drugs not yet approved in 2024 by OncoDaily
What Are the Side Effects of Zenocutuzumab?
Side effects of Zenocutuzumab are generally mild to moderate. Here’s a more detailed look at some common side effects and how they can be managed:
- Diarrhea (18%): This is a common side effect. Your doctor may recommend anti-diarrheal medications and encourage you to stay hydrated.
- Fatigue (12%): Fatigue can occur, so it’s important to rest when you feel tired. Your doctor may adjust your treatment if fatigue becomes overwhelming.
- Nausea (11%): Anti-nausea medications can help manage this side effect. Make sure to stay well-hydrated and eat small, frequent meals to reduce nausea.
Serious but Rare Side Effects: In rare cases, patients may experience reactions to the infusion like fever, chills, or breathing difficulties. These reactions are typically mild and managed by adjusting the infusion rate or giving medications to prevent them.

If you experience any of these side effects or have concerns during your treatment, it’s crucial to talk to your healthcare team immediately. They are there to help you manage symptoms and make your treatment as comfortable as possible.
How Long Will You Be on Zenocutuzumab (Bizengri)?
Treatment with Zenocutuzumab will continue until your cancer progresses (grows again) or until you experience side effects that are too difficult to manage. This could be several months, depending on how well the drug works for you.
What Other than the eNRGY trial is being conducted with Zenocutuzumab?
Zenocutuzumab is still being actively studied in clinical trials to determine its effectiveness in treating more types of cancers. Here are some ongoing studies:
-
NRG1+ NSCLC Trial (NCT05588609): Zenocutuzumab is being tested in combination with afatinib (another cancer drug) for patients with NRG1-positive non-small cell lung cancer (NSCLC). Early results show that this combination may improve response rates and progression-free survival.
-
Metastatic Castration-Resistant Prostate Cancer (mCRPC) Trial: Zenocutuzumab is also being studied in patients with metastatic castration-resistant prostate cancer (mCRPC) who have experienced disease progression. This trial is evaluating whether combining Zenocutuzumab with enzalutamide (a hormone therapy) can help control tumor growth.
These studies are important because they may expand the use of Zenocutuzumab to other types of cancer and improve treatment options for patients who haven’t responded to existing therapies.
What to Expect During Treatment
If you’re prescribed Zenocutuzumab, here’s what you can expect:
- IV Infusions: The drug will be given through an intravenous (IV) line in your arm at your doctor’s office or hospital. The treatment usually takes a few hours.
- Frequent Visits: You will need to visit your doctor every two weeks for treatment. During these visits, your doctor will monitor you for side effects and how well the treatment is working.
- Premedication: Before each infusion, you will receive medications like steroids and pain relievers to help manage any potential side effects from the infusion.
What If the Treatment Doesn’t Work?
Zenocutuzumab is not a cure for cancer, but it offers a chance to control cancer growth for patients whose tumors carry the NRG1 fusion. If the treatment isn’t effective or if you experience intolerable side effects, your doctor will discuss alternative treatment options with you. Ongoing trials and new therapies continue to emerge, so there may be other promising treatments available.
What Should You Avoid During Treatment?
- Alcohol: Drinking alcohol can worsen side effects like fatigue and nausea, so it’s best to avoid it during treatment.
- Live Vaccines: Live vaccines, like the ones for measles or yellow fever, may interfere with the treatment. Always talk to your doctor before getting any vaccines.
- Infusion Reactions: If you experience fever, chills, or difficulty breathing during your infusion, let your healthcare team know right away.
A New Option for Hard-to-Treat Cancers
Zenocutuzumab is a promising new treatment for cancers with the NRG1 gene fusion, particularly non-small cell lung cancer and pancreatic cancer. It blocks cancer cell growth signals and helps your immune system fight the cancer. While it’s not a cure, it offers an important treatment option for patients with advanced cancers that have not responded to other treatments.

You Can Read 10 most promising cancer drugs: Patient Version here
If you’re considering Zenocutuzumab, talk to your doctor about whether it’s the right choice for you. They can help guide you through the treatment process and manage any side effects that may occur.