Zenocutuzumab, a bispecific HER2/HER3 antibody, has emerged as a promising treatment for patients with NRG1 fusion-positive tumors, according to a groundbreaking study published in The New England Journal of Medicine (NEJM) in February 2025. The phase 2 eNRGy trial demonstrated durable responses across multiple tumor types, including non-small cell lung cancer (NSCLC) and pancreatic cancer, with an overall response rate (ORR) of 30%. With a favorable safety profile and significant tumor reduction observed in 72% of patients, zenocutuzumab represents a potential breakthrough for this rare oncogenic driver.
Title
Efficacy of Zenocutuzumab in NRG1 Fusion–Positive Cancer.
Authors
Alison M. Schram, M.D., Koichi Goto, M.D., Dong-Wan Kim, M.D., Ph.D., Teresa Macarulla, M.D., Ph.D., Antoine Hollebecque, M.D., Eileen M. O’Reilly, M.D., Sai-Hong Ignatius Ou, M.D., Ph.D., Jordi Rodon, M.D., Ph.D., Sun Young Rha, M.D., Kazumi Nishino, M.D., Ph.D., Michaël Duruisseaux, M.D., Ph.D., Joon Oh Park, M.D., Cindy Neuzillet, M.D., Ph.D., Stephen V. Liu, M.D., Benjamin A. Weinberg, M.D., James M. Cleary, M.D., Emiliano Calvo, M.D., Ph.D.X, Kumiko Umemoto, M.D., Misako Nagasaka, M.D., Ph.D., Christoph Springfeld, M.D., Ph.D., Tanios Bekaii-Saab, M.D., Grainne M. O’Kane, M.D., Frans Opdam, M.D., Kim A. Reiss, M.D., Andrew K. Joe, M.D., Ernesto Wasserman, M.D., Viktoriya Stalbovskaya, Ph.D., Jim Ford, M.S., Shola Adeyemi, Ph.D., Lokesh Jain, Ph.D., Shekeab Jauhari, M.D., and Alexander Drilon, M.D.,
Published in NEJM, Feb 2025
Background
Patients with advanced or metastatic solid tumors harboring NRG1 gene fusions represent a rare subset with limited targeted treatment options. The eNRGy study evaluated the efficacy and safety of zenocutuzumab, a bispecific antibody targeting HER2 and HER3, in this patient population.
Methods
This is a registrational, phase 2 clinical study. Eligible patients were ≥18 years old, had received standard therapy or were ineligible for standard treatment, and had NRG1 fusion-positive tumors confirmed via next-generation sequencing. The study followed Good Clinical Practice guidelines, with oversight from institutional review boards. The primary endpoint was overall response rate (ORR) per RECIST v1.1 by investigator assessment. Secondary endpoints included duration of response (DoR), progression-free survival (PFS), safety, pharmacokinetics, and immunogenicity.
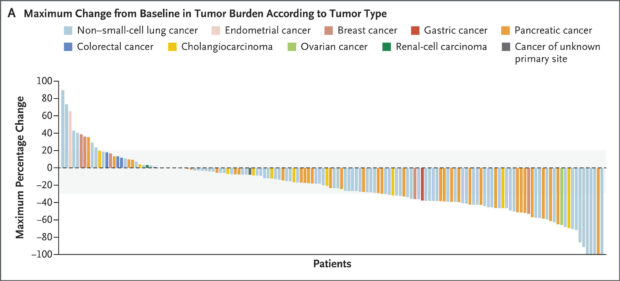
Figure from original article: Panel A shows a waterfall plot of the maximum percentage change from baseline in the tumor burden of the target lesion (sum of the longest diameters) according to investigator assessment. The upper and lower limits of the gray shading indicate 20% growth and 30% shrinkage of target lesions, respectively. Among 158 patients in the primary efficacy population with measurable disease, 10 patients discontinued without a post-baseline assessment of target-lesion response.
Study Design
A total of 204 patients across 12 tumor types received zenocutuzumab (750 mg biweekly). The primary efficacy population included 161 patients who met eligibility criteria. Tumor assessments were conducted every 8 weeks.
Results
Zenocutuzumab demonstrated promising efficacy in NRG1 fusion-positive cancers,The treatment was well tolerated, with most adverse events (AEs) being mild to moderate, and no treatment-related deaths reported. Below are the detailed results:
- Overall Response Rate (ORR): 30% (95% CI, 23–37) among 158 patients with measurable disease, including 1 complete response.
- Tumor Reduction: Observed in 72% of patients.
- Median Duration of Response (DoR): 11.1 months (95% CI, 7.4–12.9).
- Non-Small Cell Lung Cancer (NSCLC) (n=94):
- ORR: 29% (95% CI, 20–39).
- Median DoR: 12.7 months (95% CI, 7.4–20.4).
- Pancreatic Cancer (n=36):
- ORR: 42% (95% CI, 25–59).
- Median DoR: 7.4 months (95% CI, 4.0–11.2).
- Median Progression-Free Survival (PFS): 6.8 months in the overall population.
- Pancreatic Cancer Median PFS: 9.2 months.
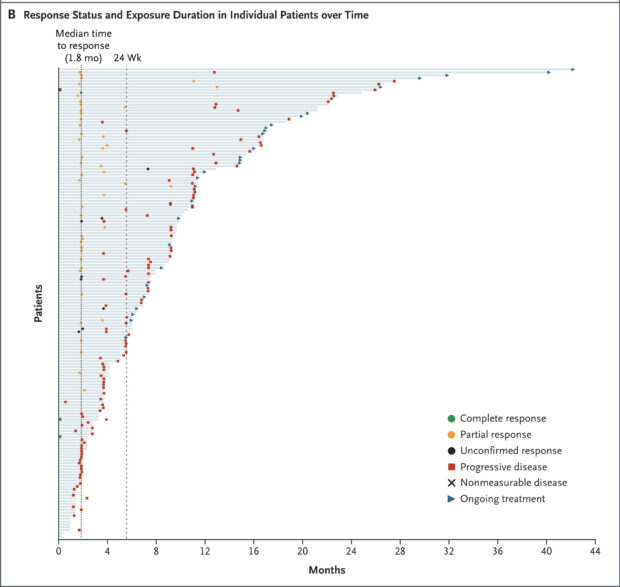
Figure from Original Article: Panel B shows a swimmer plot of outcomes, including time to response, duration of exposure, and patient status at the data-cutoff date in 161 patients in the primary efficacy population.
Safety
Zenocutuzumab was well tolerated. The most common grade ≥3 adverse events were anemia (5%), increased γ-glutamyltransferase (4%), and pneumonia (3%). Infusion-related reactions occurred in 14%, all grade 1 or 2. No treatment-related grade 3+ adverse events occurred in >5% of patients. The incidence of antidrug antibodies was 5%.
Key Findings
Zenocutuzumab demonstrated durable responses across multiple tumor types with NRG1 fusions.
- Zenocutuzumab demonstrated durable efficacy in NRG1 fusion-positive cancers, particularly in NSCLC and pancreatic cancer.
- Responses were observed across multiple fusion partners and tumor types.
- The drug exhibited a favorable safety profile, with no unexpected toxicities.
- RNA-based sequencing was confirmed as a superior method for NRG1 fusion detection.
Conclusion
The eNRGy study highlights zenocutuzumab’s efficacy and safety in patients with NRG1 fusion-positive tumors. Further research is warranted to validate these findings and expand treatment options for this rare but clinically significant oncogenic driver.
You Can Read the Full Article Here
Summary by Sona Karamyan, MD