Zenocutuzumab (Zeno, MCLA-128) is a bispecific monoclonal antibody. It targets HER2 and HER3 receptors to disrupt oncogenic signaling in tumors with neuregulin-1 (NRG1) gene fusions. On December 4, 2024, the U.S. Food and Drug Administration (FDA) granted accelerated approval to Zenocutuzumab-zbco, marketed as Bizengri, for the treatment of adults with advanced, unresectable, or metastatic non-small cell lung cancer (NSCLC) and pancreatic adenocarcinoma harboring neuregulin 1 (NRG1) gene fusions, following disease progression on or after prior systemic therapy. This approval was based on clinical trial data demonstrating the efficacy of Zenocutuzumab in targeting NRG1 fusion-positive tumors, offering a new therapeutic option for patients with these challenging cancer types.
Which company produced Zenocutuzumab
Zenocutuzumab (Zeno, MCLA-128) is developed by Merus N.V., a clinical-stage immuno-oncology company headquartered in Utrecht, the Netherlands. Founded in 2003, Merus specializes in creating innovative bispecific and trispecific antibody therapeutics designed to engage multiple targets, thereby enhancing anti-cancer effects against complex tumor mechanisms.
How does Zenocutuzumab work?
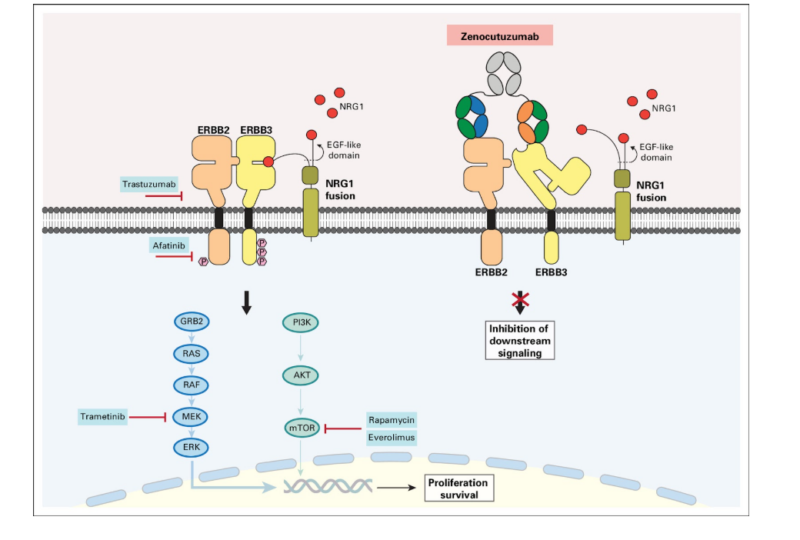
Zenocutuzumab (Zeno, MCLA-128) is designed to counteract tumor growth in cancers driven by neuregulin-1 (NRG1) gene fusions, which fuel an aggressive signaling cascade through the HER2-HER3 pathway. Under normal conditions, NRG1 binds to HER3, triggering its dimerization with HER2. This interaction activates critical oncogenic pathways, such as PI3K-AKT and MAPK, which promote uncontrolled cell growth, survival, and resistance to apoptosis—hallmarks of cancer progression. To interrupt this process, Zeno employs a unique “Dock & Block” mechanism. One arm of the antibody anchors onto HER2, securing itself to the tumor cell, while the other arm blocks HER3, effectively preventing NRG1 from binding. This dual action disrupts HER2-HER3 heterodimer formation, shutting down the oncogenic signaling responsible for tumor proliferation and survival. Beyond direct pathway inhibition, zenocutuzumab also enhances the body’s immune response through antibody-dependent cellular cytotoxicity (ADCC). By recruiting natural killer (NK) cells and other immune effector cells, it enables the immune system to recognize and destroy cancer cells more effectively. This two-pronged approach—blocking the tumor’s growth signals while activating immune-mediated killing—makes zenocutuzumab a promising therapeutic option for NRG1 fusion-positive cancers, such as non-small cell lung cancer (NSCLC) and pancreatic adenocarcinoma.
What Cancers Is Zenocutuzumab Approved to Treat?
Zenocutuzumab has received FDA approval for the treatment of advanced or metastatic non-small cell lung cancer (NSCLC) and pancreatic adenocarcinoma harboring neuregulin 1 (NRG1) gene fusions. This approval is based on clinical trials demonstrating its efficacy in these specific cancer types.
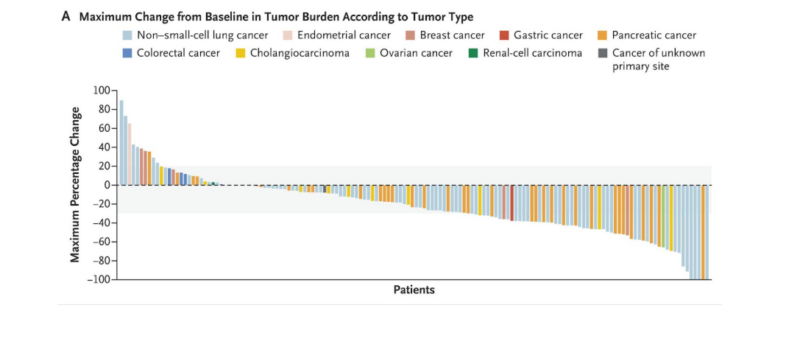
Panel A illustrates tumor burden changes from baseline, with gray shading marking 20% growth and 30% shrinkage. Among 158 patients, 10 discontinued before post-baseline assessment.
What research is behind the approval?
In the phase 2 eNRGy trial (NCT02912949), published in NEJM (Feb 2025), 204 patients with advanced NRG1 fusion-positive cancers received 750 mg of Zenocutuzumab intravenously every two weeks. Among 158 patients with measurable disease, the overall response rate was 30%, with notable activity in non-small cell lung cancer (29%) and pancreatic cancer (42%). The median duration of response was 11.1 months, and the progression-free survival (PFS) was 6.8 months, indicating sustained tumor control. Zenocutuzumab was generally well-tolerated, with mostly low-grade adverse events, including diarrhea (18%), fatigue (12%), and nausea (11%). Infusion-related reactions occurred in 14% of patients, and only one patient discontinued treatment due to side effects. For more details click here

Zenocutuzumab Combinations and Treatment Outcomes
Preclinical studies showed synergy with trastuzumab, and phase 2 trials (NCT03321981) evaluated its combination with trastuzumab and vinorelbine in heavily pretreated HER2+ MBC patients. Among 26 evaluable patients, the disease control rate was 77%, with one complete and four partial responses. The regimen was well tolerated, with common side effects including neutropenia (46% grade 3-4), diarrhea (4% grade 3-4), and no significant LVEF decline.
Zenocutuzumab side effects and its management
Zenocutuzumab has shown a favorable safety profile, with most side effects being mild to moderate and manageable with supportive care. The most frequently reported adverse events include diarrhea (18%), fatigue (12%), and nausea (11%). These symptoms can typically be controlled with hydration, dietary modifications, rest, and medications such as anti-diarrheal agents or anti-nausea drugs. Infusion-related reactions occurred in 14% of patients but were generally mild and manageable with premedication and adjustments to the infusion rate. Some patients experienced neutropenia, particularly when zenocutuzumab was combined with chemotherapy, requiring regular blood count monitoring and, in some cases, the use of granulocyte colony-stimulating factors (G-CSF).

Anemia was observed in 5% of cases and could be managed with iron supplements or erythropoiesis-stimulating agents if needed. Liver enzyme elevations were rare (4%) and required periodic monitoring, but no severe liver toxicity was reported. Importantly, no significant declines in left ventricular ejection fraction (LVEF) were observed, distinguishing zenocutuzumab from some other HER2-targeted therapies. Overall, the treatment was well tolerated, with few patients needing to discontinue therapy due to side effects. With appropriate monitoring and supportive care, zenocutuzumab remains a promising option for patients with NRG1 fusion-positive cancers.
What is the Recommended Dosage of Zenocutuzumab
Zenocutuzumab is available as a 375 mg/18.75 mL (20 mg/mL) injection solution in a single-dose vial. It is approved for NRG1 fusion-positive advanced or metastatic non-small cell lung cancer (NSCLC) and pancreatic adenocarcinoma in adults who have progressed after prior treatment. The recommended dose is 750 mg IV every two weeks, with premedication required before each infusion. Treatment continues until disease progression or unacceptable toxicity. For storage, unopened vials must be refrigerated between 2-8ºC (36-46ºF), protected from light, and must not be frozen or shaken. Once diluted, the solution can be stored for up to 6 hours at room temperature or 28 hours if refrigerated. If the infusion time exceeds these limits, a new bag must be prepared to maintain drug stability.
How is Zenocutuzumab administered?
Zenocutuzumab is administered via IV infusion and must be carefully prepared. The solution should be clear to slightly yellow—discard if discolored or contains particles. To prepare, remove 37.5 mL of saline from a 250-mL infusion bag and replace it with 37.5 mL from two vials (750 mg total dose). The bag should be gently inverted to mix the solution, but shaking must be avoided to prevent compromising the drug’s stability. Premedication is required before each dose, including dexamethasone (10 mg), acetaminophen (1000 mg), and an H1 antihistamine. Dexamethasone is optional after the first dose. If refrigerated, the diluted solution should sit at room temperature for 30 minutes before infusion. Zeno is then infused over four hours through a peripheral or central IV line, using an in-line 0.2-micron filter with close monitoring during and at least one hour post-infusion. It must not be mixed with other medications in the same IV line.
What to Avoid During Zenocutuzumab Treatment?
While undergoing Zenocutuzumab treatment, certain precautions can help ensure safety and effectiveness. Some drugs may interfere with Zenocutuzumab, either reducing its efficacy or increasing side effects. Always inform your doctor about any medications, including over-the-counter drugs, supplements, or other cancer treatments. Alcohol can worsen side effects like fatigue and nausea, so it’s best to limit or avoid it. Additionally, live vaccines, such as MMR or yellow fever, should generally be avoided due to potential immune system interactions. Always consult your doctor before receiving any vaccines. Since Zeno is given intravenously, be aware of potential infusion reactions like fever, chills, or breathing difficulties. Inform your healthcare team immediately if these occur. Regular heart function monitoring is also advised, as the drug targets HER2, which plays a role in cardiac function. Avoid excessive caffeine or high-salt diets to reduce heart strain. Zenocutuzumab may harm a developing fetus, so effective contraception is recommended during treatment and for a period afterward. Breastfeeding should be avoided, as it’s unclear if the drug passes into breast milk. Additionally, fatigue and muscle weakness may occur, so patients should listen to their bodies and avoid overly strenuous activities if feeling unwell.
Zenocutuzumab effectiveness over time
The article, published in JCO on August 17, 2022, describes a case of sustained tumor regression with Zeno in a 51-year-old woman with NRG1 fusion–positive, estrogen receptor–positive (ER+) breast cancer. The patient had previously progressed on multiple lines of therapy, including a cyclin-dependent kinase 4/6 (CDK4/6) inhibitor. After starting Zenocutuzumab, a bispecific antibody targeting HER2 and HER3 signaling, she experienced significant and durable tumor shrinkage. The treatment effectively blocked the tumor-promoting effects of NRG1 fusions, leading to prolonged disease control. This case highlights Zenocutuzumab’s potential as a promising targeted therapy for NRG1-driven cancers, even in heavily pretreated patients.
A study presented at ESMO 2023 highlighted the durable efficacy of Zeno, a HER2 × HER3 bispecific antibody, in patients with NRG1 fusion-positive pancreatic ductal adenocarcinoma (PDAC). Among 33 patients, the overall response rate (ORR) was 42.4%, with 82% experiencing tumor reduction. The median duration of response (DoR) was 9.1 months, and the treatment was well tolerated, with only 6% experiencing grade 3-4 toxicities. These findings suggest that Zenocutuzumab could become a new standard of care for this rare and aggressive cancer, addressing a significant unmet medical need.
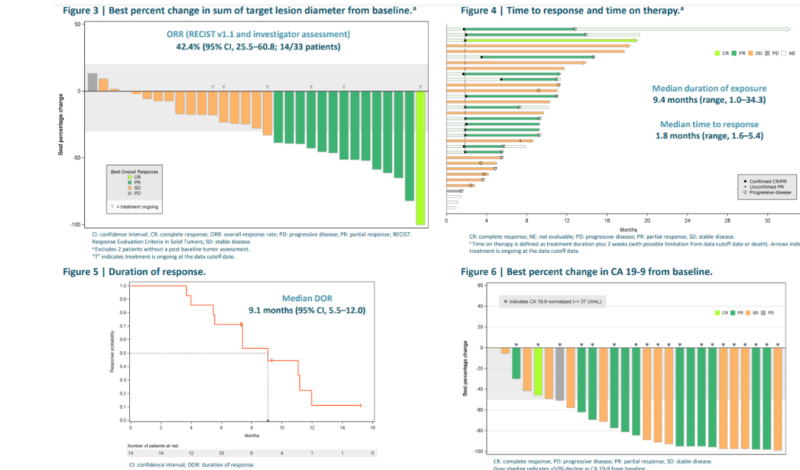
Zenocutuzumab has demonstrated promising efficacy in NRG1 fusion-positive cancers, particularly NSCLC and pancreatic cancer. In the phase 2 eNRGy trial, it achieved a 30% overall response rate (ORR), with tumor reduction in 72% of patients. The median duration of response (DoR) was 11.1 months, progression-free survival (PFS) was 6.8 months, and overall survival (OS) data is still maturing.
Ongoing trials with Zenocutuzumab
The Phase II, open-label, multicenter international study (NCT05588609) evaluates the efficacy of Zeno alone or in combination for patients with NRG1+ non-small cell lung cancer (NSCLC) and metastatic castration-resistant prostate cancer (mCRPC). In Group A, approximately 50 NRG1+ NSCLC patients receive zenocutuzumab with afatinib (40 mg daily), while Group B enrolls up to 40 mCRPC patients who continue enzalutamide or abiraterone alongside zenocutuzumab after prior disease progression. The treatment period includes an initial safety run-in phase and an expansion phase with interim efficacy analysis. The study consists of four stages: Screening, Treatment, Safety Follow-up, and Long-term Follow-up.
You can read Patient Version Here
Written by Mariam Khachatryan, MD
FAQ
What is Zenocutuzumab and how does it work?
Zenocutuzumab is a bispecific antibody that targets both HER2 and HER3, blocking their interaction and inhibiting HER2/HER3 dimerization. This prevents activation of the PI3K/AKT/mTOR pathway, which is essential for cancer cell growth and survival.
Which cancers are treated with Zenocutuzumab?
Zenocutuzumab is primarily used for NRG1 fusion-positive cancers, including non-small cell lung cancer (NSCLC) and pancreatic adenocarcinoma. Clinical trials are also exploring its potential in other cancers with HER2/HER3 involvement.
What are the most common side effects of Zenocutuzumab?
Common side effects include fatigue, nausea, diarrhea, infusion-related reactions, and neutropenia. Most are manageable with supportive care, and premedications can help reduce infusion reactions.
How is Zenocutuzumab administered?
Zenocutuzumab is given as an intravenous (IV) infusion every two weeks. The first infusion is given over four hours, with subsequent doses possibly administered more quickly depending on tolerance.
What precautions should be taken during Zenocutuzumab treatment?
Patients should avoid live vaccines, excessive alcohol consumption, and pregnancy during treatment. Regular cardiac monitoring may be required due to potential effects on heart function.
How is Zenocutuzumab stored?
Unopened vials should be stored in the refrigerator (2-8ºC/36-46ºF), protected from light, and should not be frozen or shaken. Once diluted, the solution must be used within specific timeframes depending on storage conditions.
Can Zenocutuzumab be used with other cancer treatments?
Yes, Zenocutuzumab is being tested in combination with drugs like afatinib, enzalutamide, and abiraterone for enhanced efficacy in specific cancer types.
What happens if a dose of Zenocutuzumab is missed?
If a dose is missed, the treating oncologist will determine the best course of action. Delays may impact treatment effectiveness, so adherence to the prescribed schedule is crucial.
What should patients expect in terms of treatment outcomes?
Clinical data suggest Zenocutuzumab provides promising tumor responses, particularly in NRG1+ cancers. However, individual outcomes vary, and factors such as progression-free survival (PFS) and overall survival (OS) are being continuously evaluated in trials.