Vorasidenib is a newly approved targeted therapy used to treat certain brain tumors. In August 2024, the U.S. Food and Drug Administration (FDA) approved vorasidenib for patients aged 12 and older with Grade 2 astrocytoma or oligodendroglioma that carry specific IDH1 or IDH2 mutations. This approval offers new hope for people living with these slow-growing brain tumors following surgery such as biopsy or resection.
What Is Vorasidenib and How Does It Work?
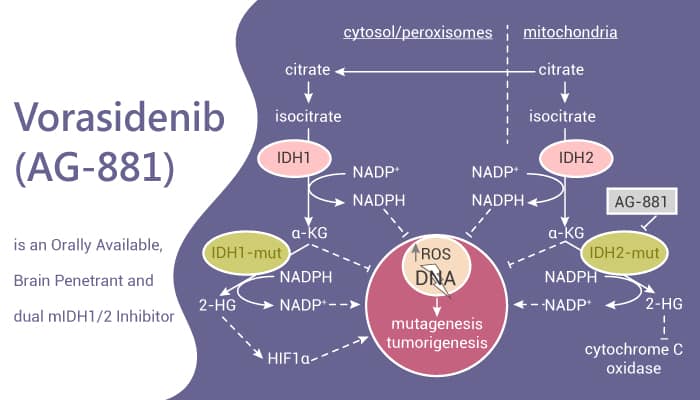
Vorasidenib, marketed as Voranigo®,is part of a class of cancer drugs called IDH inhibitors. It works by targeting abnormal versions of enzymes called isocitrate dehydrogenase 1 and 2 (IDH1 and IDH2). In healthy cells, these enzymes help with normal metabolism. But when mutated, they can produce a harmful substance called 2-hydroxyglutarate (2-HG), which disrupts how cells grow and mature—leading to tumor formation.
By blocking the mutant IDH enzymes, vorasidenib reduces 2-HG levels. This helps restore normal cell function, slows tumor growth, and may delay the need for additional treatments like chemotherapy or radiation.
What Cancers Does Vorasidenib Treat?
Vorasidenib is approved for:
- Grade 2 astrocytoma
- Grade 2 oligodendroglioma
These are types of lower-grade gliomas, which are slow-growing brain tumors. The drug is used after surgery in patients who have tumors with IDH1 or IDH2 mutations, which are found in about 10-15% of gliomas.
What Is a Clinical Trial and Why Does It Matter?
A clinical trial is a research study designed to test new drugs and treatments in patients to determine their safety and effectiveness. Before Vorasidenib was approved, it went through multiple phases of clinical trials to assess how well it worked, what side effects it caused, and whether it was better than existing treatments. Clinical trials are essential because they provide scientific evidence that a drug can help patients while ensuring it is safe for widespread use.

What Does FDA Approval Mean?
When a drug receives FDA approval, it means that after rigorous testing in clinical trials, it has been shown to be both safe and effective for treating a specific condition. This approval makes the drug widely available for doctors to prescribe and helps patients access new, cutting-edge treatments sooner.
Efficacy and Results from Clinical Trials
Vorasidenib was approved based on results from a large clinical study called a Phase 3 trial (the INDIGO trial), published in the New England Journal of Medicine in June 2023. This was a carefully designed study to test whether vorasidenib could help people with certain slow-growing brain tumors called grade 2 gliomas that have IDH mutations.
The trial included 331 patients aged 12 and older who had undergone surgery for their brain tumor. These patients had either astrocytoma or oligodendroglioma, and their tumors carried IDH1 or IDH2 mutations.
Patients were randomly given either:
- Vorasidenib (40 mg once a day)
- Or a placebo (a pill with no active drug)
The main goal was to see how long patients lived without their tumor growing or needing more treatment. This is called progression-free survival (PFS).
Key Findings
- Patients taking vorasidenib stayed free from tumor growth (PFS) for a median of 27.7 months, compared to 11.1 months for those on placebo.
- At 18 months, 86% of patients taking vorasidenib still didn’t need additional treatment, compared to only 47% on placebo.
- Vorasidenib significantly delayed the need for radiation or chemotherapy, which can cause serious long-term side effects—especially important for younger patients.
Because of these strong results, the U.S. Food and Drug Administration (FDA) approved vorasidenib on August 6, 2024, for use in patients 12 years and older with IDH-mutant grade 2 gliomas, after they’ve had surgery. This approval gives patients a new option to help manage their cancer and delay more aggressive treatments like radiation and chemotherapy.
You can read more about Brain Cancer: Causes, Symptoms, Diagnosis, and 2025 Advances in Treatment on OncoDaily.
Vorasidenib Side Effects and Management
Vorasidenib (Voranigo) is a medication used to treat certain brain tumors, but like all treatments, it can cause side effects. It’s important to understand these potential side effects and how they can be managed for the best treatment experience.
Common Side Effects
Fatigue is one of the most common side effects, often leaving patients feeling tired and needing more rest. Muscle or joint pain can also occur, affecting movement and comfort. Many patients experience gastrointestinal issues, like diarrhea, which can lead to dehydration, and constipation, which can cause abdominal discomfort. Some people may also notice a reduced appetite, which can affect nutrition. Nausea is another frequent side effect that can interfere with daily activities. Blood tests may show changes, such as elevated liver enzymes, which could indicate liver stress.
Less Common and Serious Side Effects
While rare, some serious side effects require immediate attention. Liver problems can occur, leading to symptoms like yellowing of the skin or eyes, dark urine, loss of appetite, upper abdominal pain, and fatigue. If any of these symptoms appear, it’s important to contact a doctor right away. There may also be concerns about fertility, as animal studies suggest that vorasidenib might affect fertility in both men and women. It’s a good idea to talk to a doctor about reproductive plans before starting treatment.
Managing Side Effects
Managing side effects involves regular monitoring and timely intervention. Doctors will regularly check liver function through blood tests, and if needed, may adjust the dose or pause treatment. For gastrointestinal issues like nausea and diarrhea, medications and hydration can help. If seizures occur, additional treatments like anticonvulsants may be necessary. Those concerned about fertility should consider consulting with a fertility specialist.
Staying in close contact with the healthcare team is key. If any new or worsening symptoms arise, patients should report them so that the treatment plan can be adjusted as needed.

Vorasidenib Dosage and Administration
Vorasidenib comes in 10 mg and 40 mg tablets and is used to treat Grade 2 astrocytoma or oligodendroglioma with IDH1 or IDH2 mutations. The typical starting dose for adults is 40 mg taken once daily, and treatment continues until the disease worsens or side effects become too much to manage. For children aged 12 and older, the dose depends on their weight. Those who weigh 40 kg or more take 40 mg once daily, while those under 40 kg take 20 mg once daily. Treatment also continues until the disease progresses or side effects are unacceptable.
Vorasidenib should be taken once daily, with or without food, at the same time each day. The tablet should be swallowed whole with water and not chewed or crushed. If a dose is missed, it can be taken within 6 hours of the scheduled time. If it’s been more than 6 hours, skip the dose and take the next one as usual. If a dose is vomited, don’t take a replacement dose, just continue the regular schedule. Store the medication at room temperature, away from heat and moisture.
How Vorasidenib Leaves the Body?
Vorasidenib is mostly processed in the liver, mainly by an enzyme called CYP1A2, with smaller roles from other liver enzymes. Some of it is also broken down by other natural processes in the body. It stays in the body for a long time—about 10 days—before levels start to drop. Most of the drug (85%) is passed out through stool, and a small amount (about 5%) is removed in urine. This slow and steady elimination helps doctors plan the right dosing and monitor treatment safely.
What to Avoid During Vorasidenib Treatment?
During treatment with vorasidenib, it’s important to avoid smoking, as it may reduce the drug’s effectiveness. Vorasidenib can harm a fetus, so it should not be used during pregnancy. Women who can become pregnant should use effective contraception during treatment and for at least 3 months after stopping the medication. Breastfeeding is also not recommended during treatment and for 2 months after the last dose.
Strong or moderate CYP1A2 inhibitors should be avoided, as they may increase the risk of side effects, and hormonal contraceptives should not be used, as they may be less effective. Non-hormonal contraception is advised. Patients should also be cautious about potential drug interactions with medications like phenytoin, rifampin, and certain antidepressants and inform their healthcare provider about all medications they are taking.
Long-Term Outlook and Real-Life Effectiveness
Vorasidenib is not a cure, but it can delay disease progression and extend the time before further treatment is needed. Some patients have stable disease for over two years. Long-term effectiveness may vary depending on individual factors, but the drug offers a well-tolerated, less aggressive option compared to traditional chemotherapy or radiation.
Ongoing Clinical Trials
Researchers are continuing to study how vorasidenib might work even better, especially when combined with other treatments. One ongoing study (NCT06478212) is testing vorasidenib together with a chemotherapy drug called temozolomide (TMZ). The goal is to see if the combination is safe and more effective for people with IDH1- or IDH2-mutant gliomas. The study is being done in two parts. The first part helps doctors find the right dose of the two drugs when used together, and the second part looks at how well the treatment works. Participants in the study will have regular checkups during treatment and follow-up visits every three months after the treatment ends.
Another study, called the ViCToRy trial (NCT05609994), is exploring how vorasidenib works when combined with a vaccine made to target IDH1-mutant gliomas. This trial is for adults whose tumors have come back (recurrent lower-grade gliomas). Patients take daily vorasidenib and also receive regular injections of the vaccine. The vaccine is designed to train the immune system to recognize and fight the tumor. Side effects observed so far include soreness at the injection site, mild liver changes, and rare immune system reactions. These ongoing studies may lead to even better treatment options in the future.
If you’re a healthcare provider, access the professional version here.