Vorasidenib is an investigational cancer drug that belongs to a class of medicines known as targeted therapies. It was developed to interfere with specific genetic changes that occur in some tumors, particularly mutations in enzymes called IDH1 and IDH2. These mutations can lead to the buildup of harmful substances in cells, which may drive cancer growth. By blocking the activity of these mutant enzymes, vorasidenib aims to disrupt the cancer’s underlying biology and potentially slow or stop its progression.
This article will explore what vorasidenib is, how it works, its potential uses in cancer treatment, possible side effects, dosage details, what patients might expect during therapy, and more.
Which company produced Vorasidenib?
Vorasidenib, marketed under the brand name VORANIGO®, is an experimental cancer drug initially developed by Agios Pharmaceuticals and currently owned by Servier Pharmaceuticals, a global company founded in 1954 in France. Servier focuses on oncology, cardiology, and neurology, and operates in over 150 countries.
In 2021, Servier acquired Agios Pharmaceuticals’ oncology business, which included vorasidenib. Additionally, in 2024, Royalty Pharma purchased a 15% royalty on U.S. net sales of vorasidenib from Agios for $905 million, allowing them to benefit from the drug’s commercial success.
Following FDA approval in August 2024, Royalty Pharma began receiving the full 15% royalty on annual U.S. net sales up to $1 billion and a 12% royalty on sales exceeding that amount. Agios retains a 3% royalty on annual U.S. net sales above $1 billion.
How does Vorasidenib work?
Vorasidenib is designed to treat brain tumors. It works by targeting mutations in the IDH1 and IDH2 enzymes, which are involved in normal cell metabolism. In healthy cells, IDH1 converts isocitrate into α-ketoglutarate (α-KG), essential for normal cell function. However, in cancer cells with mutated IDH1 or IDH2, the enzyme produces 2-hydroxyglutarate (2-HG) instead, which disrupts cell maturation and promotes tumor growth.
Vorasidenib inhibits these mutant enzymes, reducing 2-HG levels and allowing the cells to resume normal differentiation, slowing tumor growth. IDH mutations are found in about 10-15% of gliomas and in other cancers, such as acute myeloid leukemia (AML). By restoring normal cell processes, vorasidenib offers a targeted approach to treating cancers driven by these mutations.
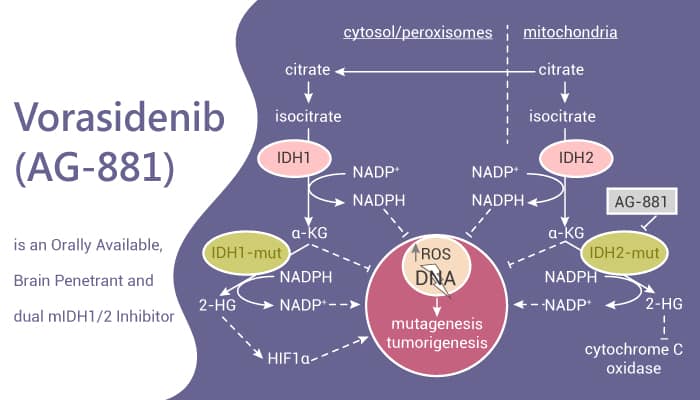
Photo from MedChemExpress official website
What Cancers is Vorasidenib Approved to Treat?
On August 6, 2024, the U.S. Food and Drug Administration (FDA) approved vorasidenib, an IDH1 and IDH2 inhibitor, for adult and pediatric patients aged 12 and older with Grade 2 astrocytoma or oligodendroglioma carrying a susceptible IDH1 or IDH2 mutation, following surgery such as biopsy, subtotal resection, or gross total resection.
What research is behind the approval?
In a Phase 3 trial published in NEJM on June 4, 2023, vorasidenib (Voranigo) significantly improved progression-free survival for patients with grade 2 IDH-mutant gliomas. The trial involved 331 patients aged 12 and older with histologically confirmed grade 2 oligodendroglioma or astrocytoma and IDH1 or IDH2 mutations. Patients received either vorasidenib (40 mg daily) or placebo, with the primary endpoint being progression-free survival (PFS). Results showed a median PFS of 27.7 months for vorasidenib, compared to 11.1 months for placebo (HR 0.39, P<0.001), and a significantly delayed need for further treatment (HR 0.26, P<0.001).
At 18 months, 85.6% of patients on vorasidenib were alive without requiring additional treatment, compared to 47.4% on placebo. Safety data revealed 22.8% of vorasidenib patients had grade 3 or higher adverse events, including elevated liver enzymes, while 13.5% of placebo patients experienced such events.
Based on these results, the FDA approved vorasidenib on August 6, 2024, for patients 12 and older with grade 2 IDH-mutant gliomas following surgery.

Vorasidenib side effects and its managemant
VORANIGO approved for treating certain types of brain tumors. While it offers therapeutic benefits, patients may experience side effects that vary in severity. Understanding these potential adverse effects and their management is crucial for optimal treatment outcomes.
Common Side Effects
In clinical trials, fatigue was one of the most frequently reported side effects, often leading to reduced energy levels and the need for more rest. Many patients experienced muscle or joint pain, which could affect mobility and general comfort. Gastrointestinal disturbances were also common, including diarrhea that sometimes led to dehydration and constipation that could be accompanied by abdominal pain, affecting digestive well-being. Some individuals noticed a decrease in appetite, which, if persistent, had the potential to influence nutritional intake.
Nausea was also a recurring issue, occasionally interfering with daily activities. In addition to these symptoms, seizures occurred in a significant portion of patients and required urgent medical attention. Blood tests during treatment commonly showed changes such as elevated liver enzymes—specifically ALT, AST, and GGT—as well as reduced neutrophil counts, pointing to possible liver stress and effects on the immune system.
Less Common and Serious Side Effects
Although less frequent, certain side effects associated with VORANIGO are more serious and require immediate medical attention. Liver-related problems were among the most concerning. These issues may present as yellowing of the skin or eyes, dark urine, reduced appetite, pain in the upper right part of the abdomen, and pronounced fatigue—signs that suggest impaired liver function. For this reason, regular monitoring of liver health through blood tests is an essential part of treatment.
Another potential concern is the drug’s effect on fertility. While definitive data in humans are limited, studies in animals have suggested that vorasidenib could affect fertility in both men and women. This highlights the importance of discussing family planning and reproductive goals with a healthcare provider before starting treatment.
Management of Side Effects
The management of vorasidenib-related side effects involves a combination of regular monitoring, prompt intervention, and supportive care. Liver function should be checked routinely with blood tests, and if significant abnormalities are detected, healthcare providers may reduce the dosage, pause treatment, or discontinue the drug altogether. Gastrointestinal symptoms like nausea and diarrhea can often be managed effectively with supportive treatments, including anti-nausea medications and hydration strategies. In cases where seizures develop, patients may need anticonvulsant therapy and an evaluation by a neurologist. For individuals concerned about fertility, early consultation with a fertility specialist can help explore preservation options and address potential reproductive risks.
Maintaining open communication with the medical team is essential. Patients are encouraged to report any new or worsening symptoms promptly so that appropriate action can be taken to ensure their safety and the best possible treatment experience.

What is the Recommended Dosage of Vorasidenib?
Vorasidenib is available in two tablet strengths: 10 mg and 40 mg. It is used to treat Grade 2 astrocytoma or oligodendroglioma with IDH1 or IDH2 mutations. The usual starting dose is 40 mg once daily, continued until the disease worsens or side effects become unmanageable.
For adults, the recommended dose of VORANIGO is 40 mg taken orally once daily. Treatment should continue until there is disease progression or the patient experiences unacceptable toxicity.
For pediatric patients aged 12 years and older, the dosage depends on their weight. Those weighing 40 kg or more should take 40 mg orally once daily, while those weighing less than 40 kg should take 20 mg orally once daily. Similar to adults, treatment should be continued until disease progression or unacceptable toxicity occurs.
If side effects occur, the dose may be reduced to 20 mg and, if needed, further reduced to 10 mg. If the lowest dose is not well tolerated, treatment should be stopped.
Liver function must be closely monitored. Mild changes in liver enzyme levels may not require stopping treatment, but more significant elevations may require pausing the medication. If liver function returns to normal within four weeks, treatment may resume at the same or a lower dose. Prolonged recovery or recurring liver issues may require discontinuation. Severe liver toxicity or elevated bilirubin levels should lead to stopping the medication permanently.
Learn more about Brain Cancer: Causes, Symptoms, Diagnosis, and 2025 Advances in Treatment on OncoDaily.
How is Vorasidenib administered?
Vorasidenib should be taken once daily, with or without food, at the same time each day. The tablet must be swallowed whole with water—do not crush, chew, or split it.
If a dose is missed, it can be taken within 6 hours of the scheduled time. If more than 6 hours have passed, skip the dose and continue with the next one as planned. If a dose is vomited, do not take a replacement dose and resume the regular schedule the next day. For storage, keep the medication at room temperature between 20–25°C, away from heat and moisture.
How Vorasidenib Is Processed and Removed from the Body?
Vorasidenib is primarily metabolized in the liver by an enzyme called CYP1A2, with smaller contributions from other enzymes like CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, and CYP3A. In addition to these pathways, up to 30% of the drug may be broken down through other non-CYP mechanisms.
Once in the body, vorasidenib has a relatively long half-life of about 10 days, meaning it stays in the system for an extended period. At steady state, the average rate at which the drug is cleared from the body is approximately 14 liters per hour. When it comes to elimination, the majority of vorasidenib—about 85%—is excreted through feces, with 56% of that being the unchanged form of the drug. Only a small portion, around 4.5%, is eliminated through the urine. This information helps guide dosing and safety monitoring during treatment.
What to Avoid During Vorasidenib Treatment?
During treatment with vorasidenib, certain precautions should be followed to maximize effectiveness and reduce side effects. Smoking should be avoided as it may lower the drug’s effectiveness.
Vorasidenib can cause harm to the fetus, so it is not recommended during pregnancy. Women of reproductive potential should use effective contraception during treatment and for at least 3 months after the last dose. Breastfeeding should be avoided during treatment and for 2 months after the last dose due to potential risks to the infant.
Strong or moderate CYP1A2 inhibitors should be avoided as they can increase vorasidenib exposure, raising the risk of side effects. If necessary, the dose may need to be adjusted. Hormonal contraceptives should also be avoided, as vorasidenib may reduce their effectiveness. Non-hormonal contraception is recommended.
It is important to be cautious of drug interactions with medications like phenytoin, rifampin, and certain antidepressants. Patients should inform their healthcare provider about all medications or supplements being taken. By following these precautions, patients can help ensure the treatment works effectively while minimizing risks.
Vorasidenib’s effectiveness over time
Vorasidenib has shown promising effectiveness in treating certain brain tumors, particularly those with IDH1 or IDH2 mutations. Clinical studies indicate that the drug can provide durable responses, with some patients experiencing disease stabilization for extended periods. The treatment continues until disease progression or if unacceptable side effects occur.
While the exact duration of its effectiveness varies from patient to patient, vorasidenib has been associated with a significant reduction in tumor growth, especially after surgery or other resection procedures. However, as with any treatment, its effectiveness may decrease over time, and close monitoring by a healthcare provider is essential to evaluate response and make adjustments as needed.
Patients may experience an initial improvement, but the drug’s long-term efficacy will depend on factors such as disease progression, side effects, and individual patient response.
Ongoing trials with Vorasidenib
A clinical trial (NCT06478212) is evaluating the safety and effectiveness of combining vorasidenib with temozolomide (TMZ) in patients with IDH1- or IDH2-mutant glioma. The study begins with a Phase 1b portion to find the recommended combination dose (RCD), followed by a Phase 2 to assess treatment efficacy. Participants will have regular study visits during treatment, with follow-up every three months after completion.
The ViCToRy trial (NCT05609994) is studying the safety and effectiveness of combining vorasidenib with the PEPIDH1M peptide vaccine in adults with recurrent IDH1-mutant lower grade gliomas. Patients receive daily vorasidenib and scheduled vaccine doses over multiple cycles, with a safety lead-in to monitor for side effects. Common side effects include injection site reactions, liver changes, and rare immune responses.
Written by Mariam Khachatryan, MD
FAQ
What is Voranigo approved for?
Vorasidenib is approved for the treatment of certain brain tumors, specifically Grade 2 astrocytoma or oligodendroglioma, in patients with IDH1 or IDH2 mutations.
How does Vorasidenib work in the body?
Vorasidenib blocks mutant IDH1 and IDH2 enzymes, which produce an abnormal substance that fuels tumor growth. By stopping this process, the drug helps slow or halt tumor progression.
Is Vorasidenib a form of chemotherapy?
No. Vorasidenib is not a chemotherapy drug—it is a targeted therapy that specifically inhibits mutated enzymes involved in glioma growth, with fewer effects on normal cells.
Can Vorasidenib be taken with food?
Yes, Vorasidenib can be taken with or without food. It should be taken once daily, at the same time each day, with water—do not crush, split, or chew the tablets.
What are the common side effects of Vorasidenib?
Common side effects include fatigue, nausea, liver enzyme elevations, headache, and changes in blood tests. Less commonly, it can cause IDH differentiation syndrome, which requires immediate medical attention.
Is Vorasidenib safe during pregnancy or breastfeeding?
No. Vorasidenib may harm a developing fetus and should not be used during pregnancy. Breastfeeding should also be avoided during treatment and for at least 2 months after the last dose.
How long do patients typically stay on Vorasidenib?
Patients remain on Vorasidenib until the cancer progresses or unacceptable side effects occur. Some patients may stay on treatment for months or years if the drug continues to work and is well-tolerated.
What happens if I miss a dose of Vorasidenib?
If a dose is missed and it’s within 6 hours of the scheduled time, take it as soon as possible. If more than 6 hours have passed, skip the dose and resume your regular schedule the next day. Do not double up.


