Tovorafenib is a targeted cancer therapy that works by blocking specific proteins involved in cancer cell growth, particularly in tumors with BRAF mutations. It is being investigated in both pediatric and adult patients with brain tumors. As part of a class of drugs known as RAF inhibitors, tovorafenib may offer a new option for patients with tumors that are resistant to standard treatments. This article will explore tovorafenib’s uses, side effects, dosage, how it works, and what to expect during treatment.
Which company produced Tovorafenib?
Tovorafenib, marketed under the brand name Ojemda, was developed by Day One Biopharmaceuticals, a U.S.-based company focused on targeted cancer therapies, especially for children with genetically defined tumors. Their goal is to create more effective and less toxic treatments for hard-to-treat pediatric cancers. In April 2024, tovorafenib received FDA approval for relapsed or refractory pediatric low-grade glioma with BRAF alterations. To expand access, Day One signed a licensing agreement with Ipsen, giving Ipsen rights to develop and commercialize the drug outside the U.S. Ipsen paid $111 million upfront (including a $40 million equity investment), with up to $350 million in milestone payments and tiered royalties. Day One retains U.S. rights and continues to lead late-stage trials.
How does Tovorafenib work?
Tovorafenib is a precision cancer therapy known as a pan-RAF kinase inhibitor, meaning it blocks several RAF proteins—ARAF, BRAF, and CRAF—that play key roles in cell growth and survival. It is specifically designed to target cancers driven by genetic mutations in the MAPK pathway, a signaling route that, when overactivated by mutations like BRAF fusions or BRAF V600 mutations, can cause cells to grow uncontrollably.
A major advantage of Tovorafenib is its ability to cross the blood-brain barrier, allowing it to reach tumors in the central nervous system—something many cancer drugs cannot do effectively. Unlike some therapies that require combination with other agents, Tovorafenib has demonstrated strong single-agent activity in cancers driven by BRAF alterations, offering a more convenient treatment option for patients with limited targeted therapy choices.
Photo from OJEMDA official website
What Cancers is Tovorafenib Approved to Treat?
The U.S. FDA granted accelerated approval to Ojemda (tovorafenib) On April 23, 2024, developed by Day One Biopharmaceuticals, for treating children 6 months and older with relapsed or refractory pediatric low-grade glioma (pLGG) that has a BRAF fusion, rearrangement, or V600 mutation. The approval is based on response rate and duration of response. Along with it, Day One received a rare pediatric disease priority review voucher.
What research is behind the approval?
The approval was based on results from the FIREFLY-1 trial, evaluated Tovorafenib (Ojemda) in 76 pediatric patients with relapsed or refractory low-grade glioma (LGG) harboring BRAF fusions, rearrangements, or BRAF V600 mutations.
The trial used the RAPNO-LGG criteria to measure the overall response rate (ORR), which was 51%. This included 36.8% partial responses (PR) and 14.5% minor responses (MR). The median duration of response (DoR) was 13.8 months.
As a secondary endpoint, the clinical benefit rate (CBR), measured by patients with stable disease (SD) lasting ≥12 months, was 57%. Descriptive analysis showed that 82% of patients had some degree of tumor shrinkage, with a 45% median reduction in tumor size from baseline. Additionally, 46% of patients had a response as early as the first radiographic scan, at approximately 6 weeks.
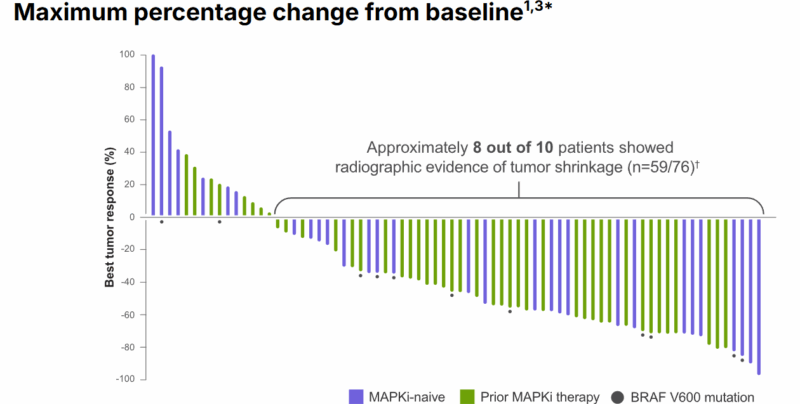
At the time of the data cutoff in June 2023, 72% of patients who had a confirmed response were still responding, with the median DoR being 13.8 months. Notably, the trial showed rapid responses to Tovorafenib, with some patients continuing treatment even after radiographic progression if clinical benefit was observed.
The most common side effects of Tovorafenib (≥30%) include rash, fatigue, hair color changes, vomiting, headache, fever, dry skin, and upper respiratory infections. Serious lab abnormalities (>2%) include decreased phosphate, hemoglobin, and albumin, and increased creatinine phosphokinase.
The recommended dose of Tovorafenib is 380 mg/m² once weekly, with a maximum dose of 600 mg. It can be taken with or without food until disease progression or intolerable side effects. Available in tablet or oral suspension form, the dose for patients with a BSA under 0.3 m² has not been established. The trial’s results were published on the Ojemda and Day One Biopharmaceuticals official websites.
Tovorafenib side effects and its management
Tovorafenib is approved for children with relapsed or refractory pediatric low-grade glioma (pLGG) that involves BRAF alterations. While this targeted treatment has shown encouraging results, it may cause side effects—some very common, others rare but more serious. Understanding these effects helps families prepare for and manage the treatment process effectively.
Common Side Effects of Tovorafenib
Most children treated with Tovorafenib experience side effects, especially during the first few months of therapy. In the pivotal FIREFLY-1 trial, the most frequently reported side effects included skin rashes, seen in about 77% of patients. Hair color changes, mostly lightening, were also extremely common, affecting 76% of children. Other typical symptoms were fatigue and viral infections, each reported in around 55% of cases.
Many children also experienced vomiting, headaches, and fevers, along with dry skin, nausea, constipation, and acne-like skin reactions. Upper respiratory tract infections were also frequently observed. These symptoms were usually mild to moderate and often managed with hydration, rest, and over-the-counter or supportive medications.
Less Common but More Serious Side Effects
Although less frequent, some side effects of Tovorafenib can be more serious and require close medical monitoring. Bleeding (hemorrhage) occurred in about 37% of children, including serious events in 5% of cases. The most common bleeding issue was nosebleeds, but one child unfortunately experienced a fatal hemorrhage.
Liver-related side effects were another area of concern. Elevated liver enzymes such as ALT and AST—a potential sign of liver inflammation—were observed in over 40% of patients. This makes routine liver function tests essential throughout treatment.
Growth delay was reported in around 15% of children. While this can be concerning, most patients resumed normal growth after stopping the medication. In addition, photosensitivity and acne-like skin rashes were relatively common, which may require sun protection and skincare support.
Laboratory monitoring often revealed low phosphate, decreased hemoglobin (anemia), and elevated creatine phosphokinase, which can indicate muscle irritation. These changes were usually not felt by the patient but were caught through regular blood tests.
Management of side effects
Tovorafenib side effects are generally manageable with proper care. Skin issues may be treated with creams or by a dermatologist. Liver enzyme elevations might require dose adjustments or short treatment breaks. Children with slowed growth should be monitored regularly, and specialist care may be needed.
Due to increased sensitivity to sunlight, patients should use sunscreen and wear protective clothing outdoors. Since Tovorafenib may harm unborn babies, effective non-hormonal birth control is essential during and after treatment for those of reproductive age.
What is the Recommended Dosage of Tovorafenib?
Tovorafenib is available as a 100 mg tablet and a 25 mg/mL oral suspension. It is approved for treating children and young adults aged six months and older with relapsed or refractory low-grade glioma (pLGG) that harbors a BRAF fusion, rearrangement, or V600 mutation.
The recommended starting dose is 380 mg/m² taken orally once a week, with or without food. The maximum dose should not exceed 600 mg per week. Treatment continues until disease progression or unacceptable toxicity occurs.
Before starting therapy, patients must be tested for BRAF gene alterations. Liver function tests and pregnancy status in females of reproductive potential should also be assessed. No dose adjustment is required for mild to moderate renal or hepatic impairment, but the drug has not been studied in cases of severe organ dysfunction.
Learn more about Brain Cancer: Causes, Symptoms, Diagnosis, and 2025 Advances in Treatment on OncoDaily.
How is Tovorafenib administered?
Tovorafenib can be taken as either tablets or an oral suspension. It is given once weekly at a regular time, with or without food. Tablets should be swallowed whole with water—do not chew, cut, or crush them.
For the oral suspension, each bottle must be reconstituted with 14 mL of room temperature water to reach a concentration of 25 mg/mL. After mixing, each bottle provides 300 mg in 12 mL. If a dose requires more than 300 mg, two bottles are used and the dose is split between them. The reconstituted suspension should be used immediately and discarded if not taken within 15 minutes. Dosing is done using the provided syringe or feeding tube (minimum 12 French).
If a dose is missed by three days or less, it should be taken as soon as possible. If more than three days have passed, skip it and wait for the next scheduled dose. If vomiting occurs right after taking the medication, the dose should be repeated.
Both tablets and the powder for suspension should be stored at room temperature (20–25ºC) in their original packaging. The suspension must be prepared fresh for each use, and any unused portion should be discarded after dosing.
How Tovorafenib Is Processed and Removed from the Body?
Tovorafenib is mainly broken down in the body by enzymes called aldehyde oxidase and CYP2C8. Other enzymes like CYP3A, CYP2C9, and CYP2C19 also help with its breakdown, but to a lesser extent.
Once processed, the drug stays in the body for about 56 hours (its half-life). It is gradually cleared from the system at a rate of 0.7 liters per hour per square meter of body surface area. Most of the drug is eliminated through feces (about 65%, with nearly 9% in its original form), and a smaller amount leaves through urine (about 27%, with almost none unchanged).
What to Avoid During Tovorafenib Treatment?
During Tovorafenib treatment, patients must take several important precautions. The drug increases sensitivity to sunlight, so it’s essential to avoid prolonged sun exposure. Wearing sunscreen and protective clothing is recommended when outside to prevent sunburn. Additionally, Tovorafenib can harm an unborn baby, making it necessary for patients of reproductive age to use non-hormonal contraception during and after treatment to prevent pregnancy.
Patients should also avoid drug interactions that may affect liver enzymes, as these can impact how Tovorafenib works in the body. It’s important for patients to inform their healthcare provider of any other medications or supplements they are taking. The drug is not recommended for patients with neurofibromatosis type 1 (NF1), as studies suggest it may accelerate tumor growth in these cases. Genetic testing before treatment helps ensure safe and appropriate use.
Lastly, patients should not stop or adjust their dosage without consulting their doctor. If side effects occur, the doctor may adjust the dose or temporarily pause treatment. Following the doctor’s guidance is crucial for achieving the best results during therapy.
Tovorafenib’s effectiveness over time
Tovorafenib has shown strong effectiveness in treating pediatric low-grade glioma (pLGG) with BRAF gene alterations. In clinical trials like FIREFLY-1, many patients experienced partial responses and stable disease for extended periods. However, the duration of effectiveness can vary, with some tumors eventually progressing despite treatment. Ongoing monitoring is essential to adjust treatment as needed. Overall, Tovorafenib offers long-term benefits for many patients, particularly those with BRAF mutations.
Ongoing trials with Tovorafenib
The clinical trial NCT06965114 evaluates the combination of Tovorafenib (DAY101) and Rituximab for treating relapsed or refractory classical hairy cell leukemia (cHCL). The study aims to assess safety, effectiveness, and minimal residual disease (MRD) rates in comparison to the current standard treatment, cladribine and rituximab. Tovorafenib targets mutated BRAF proteins, potentially stopping cancer growth, while Rituximab helps the immune system target cancer cells. The trial includes both a Phase 1b safety evaluation and a Phase 2 randomized comparison with cladribine. Key objectives are to determine the complete remission rate, progression-free survival, and long-term response in patients receiving this combination therapy.
The VICTORY study (NCT06381570) investigates the combination of vinblastine and tovorafenib in pediatric patients with recurrent or progressive low-grade gliomas (LGG) harboring RAF alterations. This pilot study has two phases: Phase A focuses on determining the maximum tolerated dose (MTD) of the combination, and Phase B aims to assess its efficacy. Patients will receive treatment for up to 24 cycles, with radiographic evaluations every three cycles. The study seeks to evaluate the safety and effectiveness of this combination in treating RAF altered LGGs in young patients.
The Phase 2 trial (NCT05828069) evaluates the effectiveness of tovorafenib (DAY101) for treating progressive, relapsed, or refractory Langerhans cell histiocytosis (LCH), a disease where excess immature white blood cells form tumors in various organs. The study aims to determine the overall response rate, adverse events, and long-term survival for patients treated with tovorafenib. It also explores the drug’s impact on tumor mutations and cell populations. Patients will receive tovorafenib for up to 12 cycles, with follow-up for up to 2 years post-treatment.
Written by Mariam Khachatryan, MD
FAQ
What is Tovorafenib used for?
Tovorafenib is used to treat children relapsed or refractory pediatric low-grade glioma (pLGG) that has a BRAF fusion, rearrangement, or V600 mutation.
Who developed Ojemda?
Tovorafenib was developed by Day One Biopharmaceuticals, a California-based company dedicated to creating precision therapies for pediatric and genetically defined cancers.
Is Tovorafenib approved for adults or only for children?
Currently, the FDA has approved Tovorafenib for pediatric patients aged 6 months and older with certain BRAF-mutated brain tumors.
What are the most common side effects of Tovorafenib?
Common side effects include rash, fatigue, hair color changes, vomiting, headaches, fever, and dry skin. Most are manageable with supportive care, but regular checkups are needed to monitor and treat any complications early.
Does Tovorafenib interact with other medications?
Tovorafenib may interact with drugs that affect liver enzyme activity. Patients should inform their healthcare provider about all medications and supplements to avoid harmful interactions.
What precautions should patients take while on Tovorafenib?
Patients should avoid sun exposure due to photosensitivity, and use non-hormonal contraception if they are of reproductive age. Routine blood tests are important to monitor for liver and blood-related side effects during treatment.
Are there ongoing clinical trials involving Tovorafenib?
Yes, Tovorafenib is being studied in additional conditions such as hairy cell leukemia, Langerhans cell histiocytosis, and in combinations with vinblastine or rituximab.
What is a BRAF mutation, and why is it important?
A BRAF mutation alters a protein that helps control cell growth. When mutated, it can cause cells to grow uncontrollably. Tovorafenib specifically targets cancers with BRAF mutations or fusions, helping block abnormal signaling and limit tumor progression.