Talha Badar, Hematologist at Mayo Clinic Comprehensive Cancer Center, shared a post on X:
”Deep dive into ASC4FIRST trial CML Asciminib in Newly Diagnosed Chronic Myeloid Leukemia.”
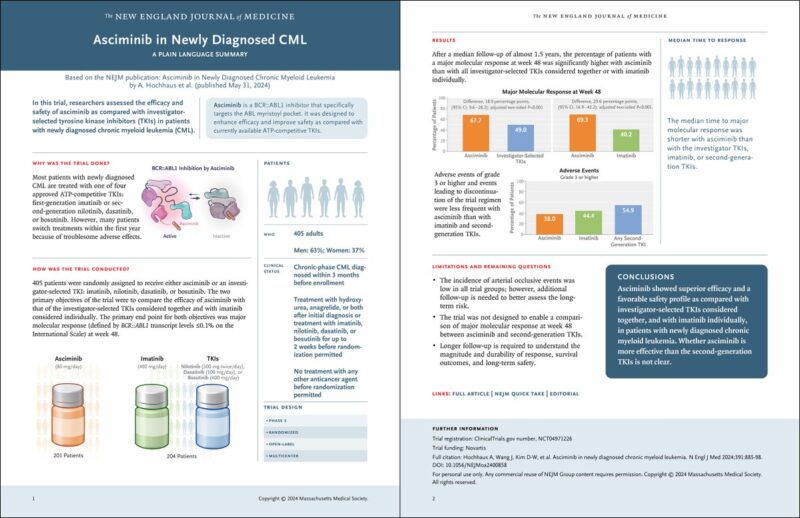
”ASC4FIRST trial
Background Imatinib: can achieve near normal life expectancy, once complete CG remission is accomplished. But associated with lower response rate compared to 2nd-3rd generation TKI and high resistance/progression.
2nd generation TKI: produce faster, deeper molecular responses, however, they are associated with more AE’s requiring dose modifications.
Asciminib has unique mechanisms of action (STAMP inhibitor) with less off target AEs.”
”ASC4FIRST trial
Method
PIII trial utilizing asciminb vs imatinib or 2ndgeneration TKI in treatment naïve CML-CP
Primary endpoint MMR defined as BCR-ABL1 ≤0.1% on IS at 48 wks.
201 received asciminib and 204 physician choice TKI
Median f/u 1.5 yrs”
”ASC4FIRST trial
The distribution of patients in the investigator selected TKI group (imatinib, 50% of patients; nilotinib, 24%; dasatinib, 21%; and bosutinib, 5%)
MMR at 48 wks 67.7% vs 49% with asciminib and physician choice TKI respectively (95% CI 9.6-28.2%, P<0.001)
Sub-group, MMR at 48 wks: 66% with asciminib vs 57.8% with 2ndgen TKI (95% CI: -5.1% to 21.5%)”
”ASC4FIRST trial
Safety
AE’s G3 or higher (asciminib 38%, imatinib 44.4%, 2ndgen TKI 54.9%) or discontinuation (asciminib 4.5% imatinib 11.1% vs 2ndgen TKI 9.8%)”
”ASC4FIRST trial
Summary:
Better tolerability and safety profile than other TKI MMR was not significantly better to 2nd generation TKI (trial was not designed to compared MMR with 2nd generation TKI? )
The trial was not design to assess survival advantage but to (1) assess AE’s profile (2) achievement of MMR that may dictate possibilities of TFR compared to imatinib and ?? 2ndgen TKI.
Safety profile suggest if may be safe in older individuals with comorbidities where 2nd gen TKI may not be ideal!
Longer follow-up needed to assess safety with continuous exposure.
Interestingly, number of acquired resistance mutations were low across all gps.
Big issue is out of pocket cost of the drug!”
”Current FDA label for asciminib
1 Newly diagnosed Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase (Ph+ CML-CP).
This indication is approved under accelerated approval based on major molecular response rate. Continued approval for this indication may be contingent upon verification of clinical benefit in a confirmatory trial(s)
(2) Previously treated Ph+ CML-CP
(3) CML-CP with T315I”
Asciminib in Newly Diagnosed Chronic Myeloid Leukemia
Authors: Andreas Hochhaus, Jianxiang Wang, Dong-WookKim, Dennis Dong Hwan Kim, Jiri Mayer, Yeow-Tee Goh, Philipp le Coutre, Naoto Takahashi, Inho Kim, Gabriel Etienne, David Andorsky, Ghayas C. Issa, Richard A. Larson, Felice Bombaci, Shruti Kapoor, Tracey McCulloch, Kamel Malek, Lillian Yau, Sophie Ifrah, Matthias Hoch, Jorge E. Cortes, and Timothy P. Hughes.

Dr. Talha Badar, MD, is a specialist in Hematology Oncology based in Mayo Clinic, Jacksonville, Florida. His primary areas of expertise include Leukemia, particularly Acute Myeloid Leukemia (AML), Myelodysplastic Syndrome (MDS), Acute Lymphoblastic Leukemia (ALL), and Bone Marrow Transplantation. Over his career, he has actively contributed to clinical research and clinical trials.


