Futibatinib is a targeted cancer treatment developed by Taiho Oncology. In 2022, it received FDA accelerated approval for adults with a specific type of bile duct cancer called intrahepatic cholangiocarcinoma (iCCA) that has changes in a gene called FGFR2. Researchers are also studying it in other cancers like esophageal, bladder, and colorectal cancers.
What Is Futibatinib and How Does It Work?
Futibatinib, sold under the brand name Lytgobi, is a targeted cancer medicine that blocks a protein called FGFR2. Some cancers grow because of changes in this protein, and futibatinib helps slow or stop the cancer by blocking its signals. It works by attaching to FGFR proteins and turning them off. This prevents cancer cells from growing and helps stop the tumor from spreading. Unlike some similar drugs, futibatinib binds more strongly and may work better for cancers with certain FGFR2 mutations, even those that resist other treatments.
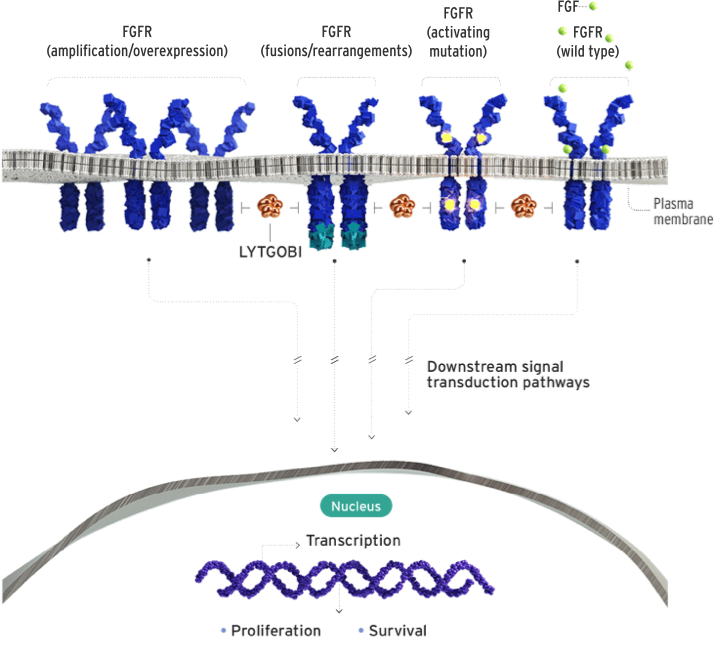
Photo from LYTGOBI official website
What Cancers Does Futibatinib Treat?
On September 30, 2022, the FDA gave accelerated approval to futibatinib, also known as Lytgobi, for adults with a specific type of bile duct cancer called intrahepatic cholangiocarcinoma (iCCA). This approval is for patients whose cancer has spread or can’t be removed with surgery and has changes in the FGFR2 gene. It is meant for those who have already received other treatments.
How Effective Is Futibatinib?
The FOENIX-CCA2 study looked at how well Lytgobi works in people with a type of bile duct cancer called intrahepatic cholangiocarcinoma (iCCA) that has FGFR2 gene changes. These patients had cancer that had spread or couldn’t be removed with surgery and had already received other treatments.
In the study, 103 patients were given futibatinib once a day. About 42% of them saw their tumors shrink, and these responses lasted for nearly 10 months on average. Most patients lived about 9 months without their cancer getting worse, and the average overall survival was nearly 22 months. The treatment worked well even in older patients and in those with other common gene changes like TP53 mutations.
The most common serious side effects were high phosphate levels in the blood, mouth sores, tiredness, and liver enzyme changes. Only a few patients had to stop treatment due to side effects, and no one died from treatment. Importantly, many patients were able to maintain their quality of life while taking futibatinib. These promising results were published in The New England Journal of Medicine in 2023 and support futibatinib as a treatment option for people with FGFR2-altered bile duct cancer.
What Is a Clinical Trial and Why Does It Matter?
A clinical trial is a research study designed to test new drugs and treatments in patients to determine their safety and effectiveness. Before futibatinib was approved, it went through multiple phases of clinical trials to assess how well it worked, what side effects it caused, and whether it was better than existing treatments. Clinical trials are essential because they provide scientific evidence that a drug can help patients while ensuring it is safe for widespread use.

What Does FDA Approval Mean?
When a drug receives FDA approval, it means that after rigorous testing in clinical trials, it has been shown to be both safe and effective for treating a specific condition. This approval makes the drug widely available for doctors to prescribe and helps patients access new, cutting-edge treatments sooner.
Recommended Dosage of Futibatinib?
The usual dose of futibatinib is 20 mg taken once a day by mouth. Each tablet contains 4 mg, so patients take five tablets daily. Treatment continues until the cancer gets worse or side effects become too serious. If side effects occur, your doctor may lower the dose to 16 mg or 12 mg per day. If you still can’t tolerate the 12 mg dose, treatment may be stopped. Futibatinib is only used in patients whose cancer has a confirmed FGFR2 gene change.
How to Take Futibatinib?
Futibatinib should be taken once a day, at the same time each day. You can take it with or without food. Swallow the tablets whole—do not crush, chew, or split them. If you vomit the dose or miss it by more than 12 hours, skip that dose and take your next one at the usual time. Do not take an extra dose to make up for it. Keep the tablets stored at room temperature 20°C to 25°C.
Futibatinib Side Effects and How to Manage Them
Futibatinib, like many targeted cancer treatments, can cause side effects. These may vary from person to person—some people experience only mild symptoms, while others may have more bothersome reactions. Knowing what to expect and how to manage these side effects can make a big difference in how patients feel during treatment and how well they tolerate it. Staying in close contact with the healthcare team is important, as early support can prevent small problems from becoming serious.
Common Side Effects
One of the most common side effects of futibatinib is hyperphosphatemia, which means high levels of phosphate in the blood. This can lead to muscle cramps, joint pain, or a tingling sensation. It is usually picked up through regular blood tests and can often be controlled with a combination of dietary changes and medications.
Fatigue is another frequent side effect. Many people report feeling unusually tired or lacking energy while on treatment. Nausea, diarrhea, and a reduced appetite are also common and may come and go. Some patients develop stomatitis, which causes mouth sores that can make eating or speaking uncomfortable. Blood tests may also show higher-than-normal liver enzyme levels, which don’t always cause symptoms but are important for doctors to monitor. A skin rash may also appear in some individuals.
Less Common Side Effects
While not as frequent, some patients experience eye-related issues, such as dry eyes or blurry vision. Changes in blood sugar levels or imbalances in other minerals and electrolytes may occur as well. Although less common, infections can happen, especially in those whose immune systems are affected. Some people may notice changes in their skin, including inflammation or sensitivity.
Managing Side Effects
Managing side effects is an important part of staying well during futibatinib treatment. High phosphate levels are usually managed with a special diet low in phosphate and the use of medications called phosphate binders. Regular blood tests help track these levels over time.
Fatigue can often be improved with good rest, staying hydrated, gentle physical activity when possible, and addressing any contributing factors. Nausea and diarrhea are typically managed with medication and dietary adjustments to keep symptoms under control. For mouth sores, maintaining good oral hygiene and using special mouth rinses or topical treatments can provide relief and help healing. Regular eye checkups are recommended, and any changes in vision should be reported to the healthcare team right away.
Above all, it’s important for patients to speak up about any symptoms they notice. Even if something feels minor, early attention can prevent it from becoming more serious. By working closely with their care team, patients can better manage side effects and continue treatment as comfortably as possible.
What Should You Avoid During Treatment?
While taking futibatinib, avoid grapefruit and grapefruit juice, as they can increase side effects. Don’t take any new medications or supplements without asking your doctor, since some can affect how futibatinib works.
To help manage phosphate levels, you may need to limit foods like cheese, milk, and soft drinks. Avoid getting pregnant during treatment and for some time after—it’s not safe for pregnancy or breastfeeding. Always swallow the tablet whole—do not crush, chew, or split it. If you miss a dose by more than 12 hours or vomit it, skip that dose and take the next one at your usual time.
What Is the Long-Term Outlook?
Futibatinib is not a cure, but it offers meaningful tumor control for many months, especially in cancers with FGFR2 changes. In the cholangiocarcinoma study, some patients lived nearly two years after starting treatment. Researchers are working to understand who benefits most and how long the effects can last. In the future, it may be combined with other drugs to make treatment even more effective.
Real-World Effectiveness
Real-life results are encouraging and reflect the success seen in clinical trials. Patients often report good quality of life during treatment, with side effects being manageable in most cases. A clinical study tested the combination of Lytgobi with pembrolizumab (an immunotherapy drug), sometimes along with chemotherapy, in patients with advanced esophageal cancer.
For patients who had not received any previous treatment, about 68% saw their tumors shrink, and nearly 90% had their disease controlled for some time. On average, the positive response lasted about 5 and a half months. In one case, the tumor shrank enough to be removed with surgery.
For patients who had been treated before but not with immunotherapy, about 43% had tumor shrinkage, and 71% had disease control, with responses lasting around 16 months on average. For those whose cancer did not respond to prior immunotherapy, fewer patients (about 6%) saw tumor shrinkage, and about half had disease control.
The most common side effects were high phosphate levels in the blood, low white blood cell counts, mouth sores, diarrhea, and changes in nails. Most side effects were manageable, and only one patient had a serious mouth sore that limited treatment. Overall, the study showed that combining futibatinib with pembrolizumab, with or without chemotherapy, is a promising and generally safe treatment approach for esophageal cancer.
Learn more about Esophageal Cancer: Symptoms, Causes, Stages, Diagnosis and Treatment on OncoDaily.
A 2025 study looked at using futibatinib with durvalumab before surgery in patients with muscle-invasive bladder cancer who couldn’t receive cisplatin. The treatment was given for three cycles before bladder removal. The goal was to increase the number of patients with no remaining cancer at surgery. The study also looked at how safe the combination is, how well it shrinks tumors, and how long patients live without the cancer coming back. Researchers are also studying how the immune system and tumor changes may affect response.
Learn more about Bladder Cancer: Symptoms ,Causes, Stages, Diagnosis and Treatment on OncoDaily.
Ongoing Research and Clinical Trials
An ongoing study called the FUTURE trial is testing a new combination treatment for people with colorectal cancer. In this trial, patients receive futibatinib along with chemotherapy (called mFOLFOX) and an immunotherapy drug (tislelizumab). The treatment lasts up to 12 months or until the cancer worsens or side effects become too strong. The goal of the trial is to see how well the treatment works, how long it helps patients live, how safe it is, and whether certain markers in the body can predict who will benefit most.
If you’re a healthcare provider, access the professional version here.